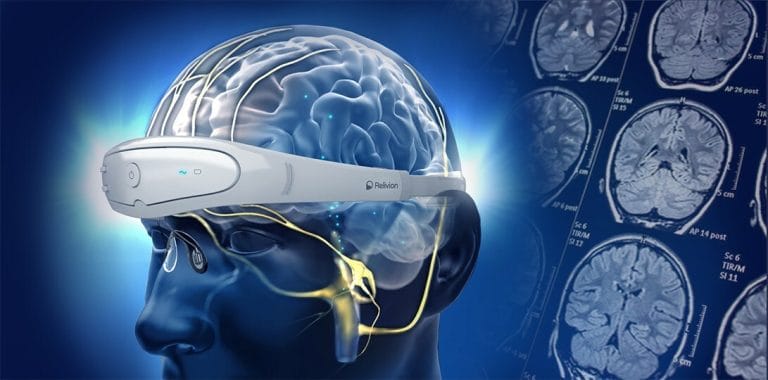
Neurolief, a leader in medical devices for neurological and neuropsychiatric disorders, has received a strategic equity investment from Sawai Group Holdings Co., Ltd. This investment strengthens the collaboration between Neurolief and the Japanese pharmaceutical corporation, who have an exclusive agreement for the development and marketing of Neurolief’s Relivion® therapy in Japan. Scott Drees, CEO of Neurolief, expressed his enthusiasm for the investment, emphasizing the shared vision to treat migraine and depression patients in Japan.
Mitsuo Sawai, CEO of Sawai Group Holdings, highlighted their commitment to improving patient care and expanding treatment options for migraine and depression through the introduction of Relivion® for at-home treatment under doctor supervision. Relivion® has recently been approved in Japan for migraine treatment, and preparations are underway for its application in depression treatment.
The Relivion® was recently approved in Japan for treatment of migraine. An application for treatment of depression is in preparation.