Hemodialysis ground-breaking results
The latest results from a clinical trial presented today at the Veith Symposium in New York City demonstrate how new living blood vessels created through restorative medical devices have become reality and can open unthinkable treatment avenues for patients. Xeltis is developing cardiovascular devices, namely artificial vessels and valves, that gradually create living and long-lasting vessels made of patients’ own, new healthy tissue.
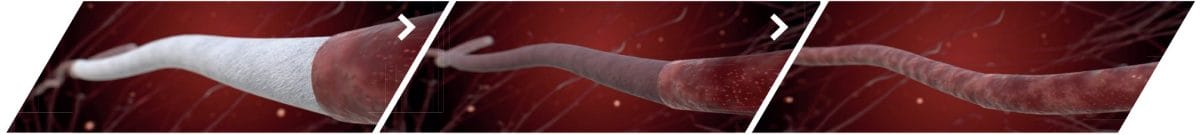
Our flagship device, aXess, is a vascular access conduit for hemodialysis in patients with end-stage kidney disease (ESKD). aXess is an investigational device currently in clinical trials in Europe. A vascular access connects the hemodialysis machine to the patient’s blood stream and is created through surgery in their body.
The results presented today at the Veith conference showed how our aXess conduit enabled over 2,400 successful dialysis sessions in a year, after having been implanted in 20 patients across Europe, as part of the ongoing First-In-Human (FIH) clinical trial. Within the first year from implant, patients had no infections and the new vessels remained fully functional for the purpose (100 percent secondary patency).
These extraordinary results were made possible because the device turned into a living vessel overtime. The new living vessels did not present any infections or other risks that are generally associated with plastic or ePTFE grafts, normally leading to their frequent replacement.
Our groundbreaking implants are a potential paradigm shift for the treatment of several major life-threatening diseases, in particular for cardiac and vascular reconstructions.
Endogenous Tissue Restoration Technology
Xeltis restorative devices are based on the proprietary Endogenous Tissue Restoration (ETR) technology, driven by Nobel prize-winning breakthroughs in polymer science. Our company is currently developing products in three clinical applications: in addition to aXess, the hemodialysis vascular access conduit, we are developing a coronary artery bypass grafting (CABG) conduit and a pulmonary valved conduit for pediatric cardiac surgery.
Our ETR platform is versatile and can be virtually applicable to all types of vascular indications. The initial clinical experience with the aXess conduit shows advantages in patency and infection rates, which may also be beneficial for several other conditions currently without an ideal solution, or without a solution at all.
In addition to our initial applications, conditions such as peripheral arterial disease (PAD), for instance, may significantly benefit from devices that enable improvement in patency and infection rates, compared to current solutions such as heterologous ePTFE.
Hemodialysis – unsolved
There is a large clinical unmet need in hemodialysis. End-stage kidney disease (ESKD) patients requiring hemodialysis are exponentially increasing in numbers worldwide.1 The economic burden for the payors is consequently becoming larger, with costs of up to US$100,000 per hemodialysis patient annually.2
The current solutions for vascular access still involve high complication rates. Arteriovenous fistulas (AVFs) remain the first-line option but require a long mean time to maturation (3.5 months) and have low success rate (26 percent at six months).5 Arteriovenous grafts (AVGs) are generally considered a second line approach3 due their inferior primary and secondary patency rates, and to the higher incidence of interventions and complications.4
The 2019 Kidney Disease Outcomes Quality Initiative (KDOQI) vascular access guideline update introduced a more patient-centric approach to vascular access choice, calling for the “right access, in the right patient, at the right time, for the right reasons.”6 With none of the existing solutions being ideal, alternative approaches are clearly very much in clinical demand.
aXess, the hemodialysis conduit
Our aXess hemodialysis vascular access conduit is a novel, restorative, bioabsorbable implant made of electrospun supramolecular polymers. Its manufacturing and chemical characteristics allow the body to heal itself.
Rather than triggering a pro-inflammatory response, like other synthetic materials, the Xeltis biomaterial enables a pro-healing neo-tissue remodeling response.7 As the neo-tissue matures, it takes over the functionality of the implant, which gradually resorbs, thereby alleviating the foreign body reaction observed with non-degradable devices.8
Over time, the aXess conduit provides a mechanical and structural scaffold for tissue cell growth promoted by the body’s natural healing process, turning into a living vessel.
aXess conduit clinical experience
The clinical trial evaluation of the aXess conduit started with the aXess first-in-human (FIH) trial (NCT04898153)—a single-arm, prospective, multicenter trial assessing safety and effectiveness of the device. In this trial, 20 patients were successfully enrolled in six European sites in Belgium, Italy, Latvia and Lithuania, between June 2021 and September 2022.
The outstanding 12-month data presented by Professor Frans Moll at the 50th Veith Symposium in New York confirmed the promising preliminary results from the first 6-month follow-up. The device showed 100 percent secondary patency, 78 primary assisted patency percent and no infections one year after implant, which are outstanding results compared to existing options. The FIH study outcomes imply significant health economics benefits, which according to company preliminary analysis, include savings for 12-21 thousand US dollars per patient in the first year post implant.
It has therefore potential to combine the short-term benefits of an AVG, including no maturation time, with the mid to long term benefits of a fistula.
Following the very promising FIH clinical outcomes, Xeltis has initiated the aXess EU pivotal trial (NCT05473299), as a go-to-market multicenter trial. Primary endpoints include freedom from device-related serious adverse events and primary patency at six months. The objective is to enroll 110 patients in up to 25 European sites and to follow them up for five years. Enrollment is well underway, with over a third of patients already implanted.
The initiation of a pivotal trial for the aXess conduit is promising for the future of hemodialysis vascular access, as its core technology may potentially combine the optimum characteristics of currently available options to offer patients, physicians and payers a solution for a major unmet need.
XABG – the next step in ETR platform
In parallel to the aXess conduit clinical development, Xeltis is progressing at FIH stage with its XABG conduit for CABG surgery, based on the same ETR platform technology. Xeltis’ solution aims to potentially make vein harvesting obsolete, reducing complication rates and healthcare system costs. CABG is the most common cardiac surgery performed worldwide, with approximately one million operations every year, using on average two vein grafts and costing USD 318 billion in the US alone each year.
The great saphenous vein (GSV) is widely used as a conduit for CABG. However, up to 18 percent of patients show complications from vein harvesting, such as surgical site infection, wound gap, bleeding, hematoma, edema, serous discharge, pain, and erythema.9 Furthermore, GSV bypass failure rates can be as high as 25 percent in the first 12 to 18 months post-surgery.10 One potential cause for this is a highly variable vein quality.11 Moreover, these changes cannot be reliably identified preoperatively, as 91 percent of GSVs are assessed by doppler ultrasound.
Leg wound infections following saphenous vein graft (SVG) harvesting represent a significant cause of prolonged hospital stay and increased costs. Around one in four patients complain about complications at harvesting site and site infections presents a median re-hospitalization stay of seven days.12
Hospitals, insurers and healthcare services have a strong interest in a better solution for CABG that eliminates the need for leg surgery to harvest vein grafts and all the subsequent complications, infections and readmissions costs. An off-the-shelf conduit that eliminates GSV harvesting and the related complications, and that improves patency outcomes is very much in need in the clinic.
The future of ETR
The recently published BEST-CLI trial13 demonstrated that alternative surgical conduits for PAD are very much in need, as a surgical treatment with a good quality conduit is superior to endovascular therapies in this challenging indication.
The ETR polymer technology is therefore at the forefront of innovation in restorative cardiovascular treatment, with the mission to bring long lasting and lifesaving solutions to patients across cardiovascular conditions that are still missing an ideal solution.
References
- Thurlow J S, Joshi M, Yan G et al. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am J Nephrol. 2021; 52(2): 98–107. doi: 10.1159/000514550.
- Swaminathan S, Mor V, Mehrotra R et al. Medicare’s payment strategy for end-stage renal disease now embraces bundled payment and pay-for-performance to cut costs. Health Aff (Millwood). 2012; 31(9): 2051–8.
- Murea M, Geary R L, Davis R P et al. Vascular access for hemodialysis: A perpetual challenge. Semin Dial. 2019; 32(6): 527–34.
- Almasri J, Alsawas M, Mainou M et al. Outcomes of vascular access for hemodialysis: A systematic review and meta-analysis. J Vasc Surg. 2016; 64(1): 236–43.
- Bylsma L C, Gage S M, Reichert H et al. Arteriovenous Fistulae for Haemodialysis: A Systematic Review and Meta-analysis of Efficacy and Safety Outcomes. Eur J Vasc Endovasc Surg. 2017; 54(4): 513–22.
- Lok C E, Huber T S, Lee T et al. National Kidney Foundation. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am J Kidney Dis. 2020; 75(4 Suppl 2): S1–S164.
- Cramer M, Chang J, Li H, Serrero A, El-Kurdi M, Cox M, et al. Tissue response, macrophage phenotype, and intrinsic calcification induced by cardiovascular biomaterials: Can clinical regenerative potential be predicted in a rat subcutaneous implant model?Tissue response, macrophage phenotype, and intrinsic calcification induced by cardiovascular biomaterials: Can clinical regenerative potential be predicted in a rat subcutaneous implant model? J Biomed Mater Res A 2022;110:245-256.
- Marzi J, Munnig Schmidt EC, Brauchle EM, Wissing TB, Bauer H, Serrero A, et al. Marker-independent monitoring of in vitro and in vivo degradation of supramolecular polymers applied in cardiovascular in situ tissue engineering. Front Cardiovasc Med 2022;9:885873.
- Sharma M, Fakih MG, Berriel-Cass D, et al. Harvest surgical site infection following coronary artery bypass grafting: risk factors, microbiology, and outcomes. Am J Infect Control. 2009;37:653–7.
- Hess CN, Lopes RD, Gibson CM, Hager R, Wojdyla DM, Englum BR, Mack MJ, Califf RM, Kouchoukos NT, Peterson ED, Alexander JH. Saphenous vein graft failure after coronary artery bypass surgery: insights from PREVENT IV. Circulation. 2014 Oct 21;130(17):1445-51.
- Giannoukas AD, Labropoulos N, Stavridis G, Bailey D, Glenville B, Nicolaides AN. Pre-bypass quality assessment of the long saphenous vein wall with ultrasound and histology. Eur J Vasc Endovasc Surg. 1997 Jul;14(1):37-40.
- Siddiqi M. Saphenous Vein Harvest Wound Complications: Risk Factors, Identification, Prevention, and Management. Chronic Wound Care Management and Research. 2016:3 147-156.
- Farber A, Menard MT, Conte MS, Kaufman JA, Powell RJ, Choudhry NK, Hamza TH, Assmann SF, Creager MA, Cziraky MJ, Dake MD, Jaff MR, Reid D, Siami FS, Sopko G, White CJ, van Over M, Strong MB, Villarreal MF, McKean M, Azene E, Azarbal A, Barleben A, Chew DK, Clavijo LC, Douville Y, Findeiss L, Garg N, Gasper W, Giles KA, Goodney PP, Hawkins BM, Herman CR, Kalish JA, Koopmann MC, Laskowski IA, Mena-Hurtado C, Motaganahalli R, Rowe VL, Schanzer A, Schneider PA, Siracuse JJ, Venermo M, Rosenfield K; BEST-CLI Investigators. Surgery or Endovascular Therapy for Chronic Limb-Threatening Ischemia. N Engl J Med. 2022 Dec 22;387(25):2305-2316.
