New data just published online in the prestigious journal JAMA Neurology has demonstrated the safety and efficacy of EASEE®, the world’s first minimally invasive brain pacemaker, in patients with focal epilepsy resistant to drug therapy.
In a meta-analysis of two identical studies involving a total of 33 patients at seven European epilepsy centres, EASEE was implanted for an eight-month evaluation period in a prospective non-randomized trial format.
After six months’ active treatment, 53% of patients demonstrated a full response to the device, as defined by a reduction in seizure frequency of at least 50% below baseline. 84% of patients demonstrated a response of some kind. There were no serious events reported which were related to either the device or its implantation.
“Results from this groundbreaking study suggest that focal cortex stimulation with an epicranial electrode array may offer a safe and effective new treatment option for patients with drug-refractory focal epilepsy”, commented Principal Investigator Professor Andreas Schulze-Bonhage, Head of the Epilepsy Centre at University Hospital, Freiburg, Germany. “An effective reduction in seizure frequency suggests that focal cortex stimulation represents a promising treatment option for patients with a predominant epileptical focus”.
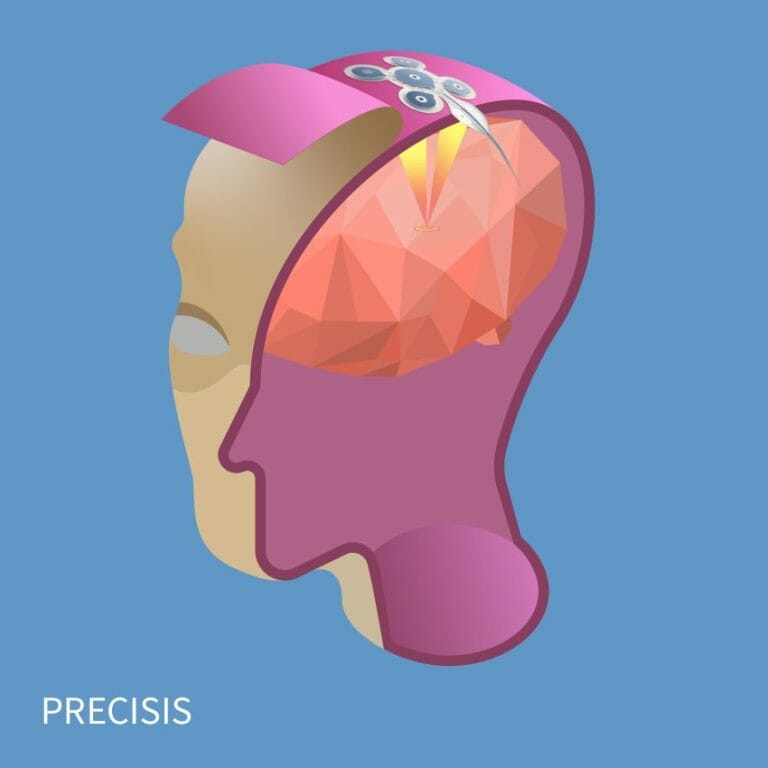
EASEE is an acronym for Epicranial Application of Stimulation Electrodes for Epilepsy. It is a system for individualized brain stimulation, which is surgically placed under the scalp, precisely over the origin of the epilepsy in the brain. This means that the cranial bone is not opened, and the brain itself remains untouched. EASEE is based on a dual mode of action: a disruptive, acute effect with high-frequency pulses every two minutes, to combat emerging seizures; and a continual current applied once a day for 20 minutes that regulates over-excitable brain areas in the long term.
EASEE is the first commercial product to be developed by Heidelberg-based Precisis, a company which specialises in the development of device-controlled therapies for the brain. EASEE was awarded an FDA Breakthrough Device Designation in February 2022, and its CE Mark in September 2022.
In October 2022 Precisis was among only 78 high-profile start-ups and SMEs in Germany, from a total of 1,000 entries, to be selected for a highly competitive grant from the European Innovation Council (EIC). In the case of Precisis the grant was in the region of 2.5 million Euros.
In February 2023 Precisis was voted one of the top 100 medium-sized companies in Germany by the prestigious Top 100 Group, on the basis of its specific innovatory strength and above-average innovation success. The innovation shown must have been demonstrated to routinely follow a strategy.
Reference:
https://jamanetwork.com/journals/jamaneurology/fullarticle/2802793