Nikon Instruments Inc. announced the release of the ECLIPSE Ni-L upright microscope which features specially balanced LED illumination with properties similar to natural light. This results in more accurate displays of the original color rendition of the specimen, making it particularly effective for the observation of pathological specimens and cells.
Release Overview
|
Product Name |
ECLIPSE Ni-L Upright Microscope |
|
Target Release Date |
May 23, 2023 |
Development Background
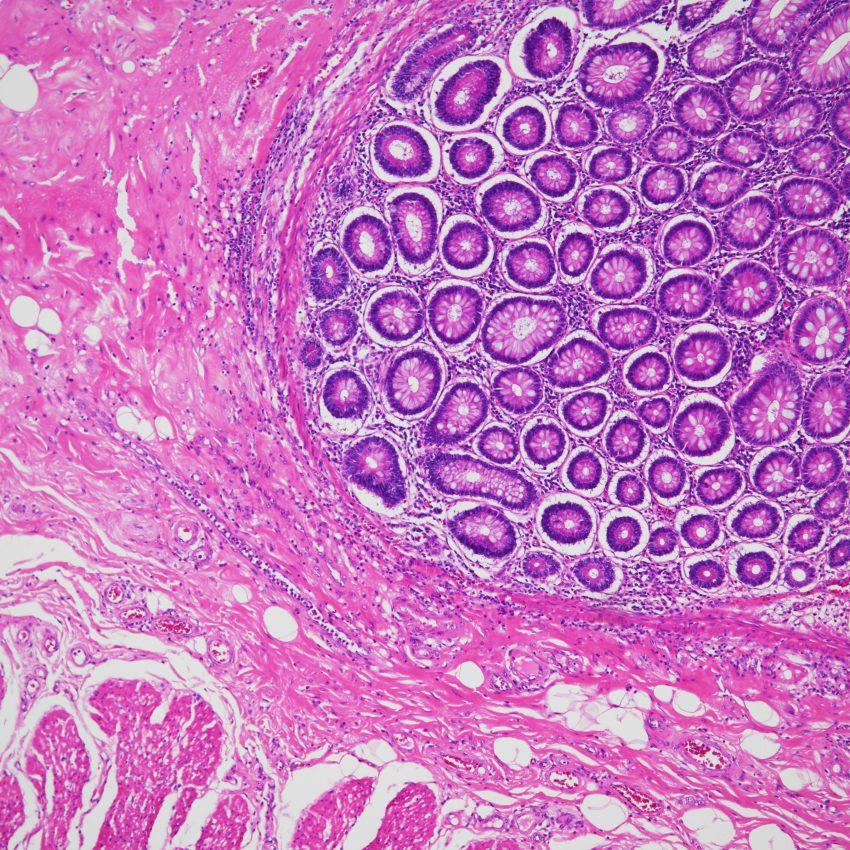
As commonly used brightfield microscopes transition away from halogen bulbs to LED light sources, this gives rise to images with blueish hues due to the unnatural color temperatures of many LED light sources. Nikon has developed the ECLIPSE Ni-L upright microscope equipped with a more balanced natural color LED light source that renders truer microscope images.
Main Features
1. High color reproducibility by the adoption of high color-rendering LEDs
Since the color of LED light is stable, color information and images can be achieved with high color reproducibility when imaging specimens using microscope digital cameras and NIS-Elements imaging software. This reduces the need to correct color by color management software and contributes to improved accuracy of image analysis and AI learning.
2.Significant reduction in environmental impact
LED light sources boast a lifespan of approximately 25 times greater*1 than that of halogen light sources, contributing to the reduction of waste materials associated with replacement. Their low power consumption also contributes to the reduction of CO2 emissions within our environment. LED light sources also significantly reduce the maintenance load in terms of light source replacement and potential downtime.
*1 Approx. 50,000 hours, estimated by Nikon standards
3. Eliminates the need to utilize color balance filters
For microscopes using a halogen light source, color adjustments are made using NCB (Neutral Color Balance) filters to correct for the varied color temperatures normally experienced with this technology. Color balanced natural light LEDs eliminate the need to use these filters and therefore also eliminate the risk of human error, such as forgetting to attach or detach color correction filters during critical imaging sessions.
4.Space-saving compact design
Microscopes using a halogen light source are designed with externally attached lamp housings in order to facilitate regular light source replacement. The ECLIPSE Ni-L, due to longer life expectancy of LED light sources, has the illumination built-in resulting in an overall size reduction of 150 mm. This increases the flexibility to choose the most effective observation location to suit your needs.
Main Specifications
|
Optical system |
CFI60 infinity optical system |
|
Illumination |
Built-in high color-rendering LED light source*2 50,000 hours of life*3 |
|
Eyepieces |
・CFI 10X ・CFI 12.5X ・CFI 15X ・CFI UW 10X |
|
Tubes |
Ergonomic binocular tube: Inclination angle 10° to 30°, extension up to 40 mm |
|
Nosepieces |
Manual/motorized nosepieces |
|
Stages |
Manual stages |
|
Condensers |
Manual condensers |
|
Observation method |
Brightfield, epi-fluorescence, phase contrast, differential interference contrast, simple polarization, sensitive color polarization, darkfield |
|
Weight |
20 kg |
|
Dimensions (mm) |
W 320 x H 492 x D 383 (brightfield configuration with ergonomic binocular tube) |
*2 Cannot be used for IR-DIC observation
*3 Estimated value based on Nikon standards

