December 14, 2020
Northwell Health, after nearly 10 months of managing an illness that has killed nearly 300,000 and sickened more than 15 million Americans, today took a major step toward the end of the COVID-19 pandemic.
Northwell Health system made history by vaccinating the first person in the United States against COVID-19, when it injected Pfizer Inc.’s medication into Sandra Lindsay, an intensive care nurse at Long Island Jewish Medical Center.
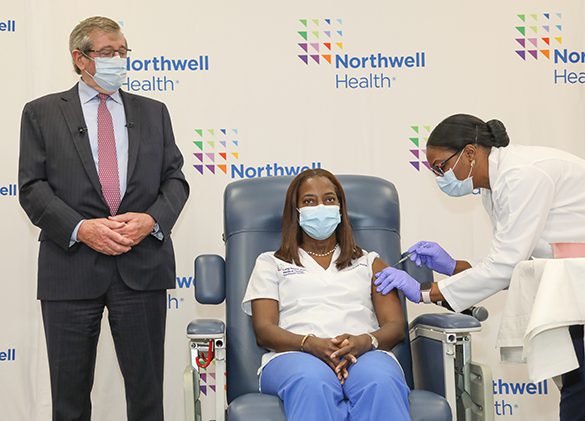
Ms. Lindsay’s participation kick-started a long-anticipated vaccination deployment program throughout the US, as well as the first phase of Northwell’s three-stage rollout to essential frontline hospital personnel. Physicians, nurses and any staff member working in direct contact with COVID-19 patients will also soon receive the first of the two-dose regimen.
“Today is V-Day in our fight against COVID-19,” said Michael Dowling, Northwell president and CEO. “This truly is a historic day for science and humanity, one in which we here in New York and across the United States have been waiting for quite some time.”
After receiving the injection, Ms. Lindsay said: “I feel great. It didn’t feel any different that when receiving the annual influenza vaccine. I would like to thank all the frontline workers, all my colleagues who’ve been doing a yeoman’s job to fight this pandemic all over the world. I feel hopeful today.
“I feel relieved. I hope this marks the beginning to the end of a very painful time in our history. I want to instill public confidence that the vaccine is safe. We’re in a pandemic and so we all need to do our part to put an end to the pandemic and to not give up too soon. There’s light at the end of the tunnel.”
Northwell Health received a limited supply of a few thousands doses — to be spread among eight hospitals — of Pfizer’s vaccine, which will require two injections 21 days apart. The vaccine has demonstrated 95-percent efficacy against infection with minimal side effects, and works on messenger RNA (mRNA) technology, which has been in development for several years. mRNA instructs cells in the body to make different proteins.
To vaccinate team members, Northwell Health has prepared a three-phase prioritization matrix to help deploy the vaccine to its over 74,000 team members. The plan factors in a person’s work/geographic area, department specialty, job function and age.
To prepare, Northwell Health invested in procuring more than 20 -70F freezers, which can store about 250,000 doses each. Northwell also stocked up on extra needles, gloves, swabs to help distribute the vaccine once ready, and the health system has been working hand-in-hand with state and federal officials for a rollout.
“This is a major milestone in our battle against COVID-19 and a remarkable effort by our researchers, scientists and health care providers,” said Mark Jarrett, MD, Northwell’s chief quality officer and deputy chief medical officer. “Our detailed plan will push the seamless vaccination of these brave health care workers. We are very excited to enter the final stage of this pandemic.”
Mr. Dowling added: “COVID-19 took our loved ones, disrupted our lives and forced us to deal with unthinkable circumstances. But hope brings prosperity and we never ended our fight. We never did wave the white flag.”
