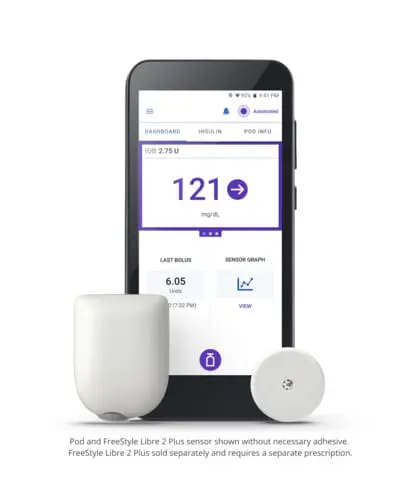
Omnipod 5
Insulet Corporation (NASDAQ: PODD) (Insulet or the Company), the global leader in tubeless insulin pump technology with its Omnipod® brand of products, today announced it has received CE mark approval under the European Medical Device Regulation for the added compatibility of the Abbott FreeStyle Libre 2 Plus sensor with Insulet’s Omnipod 5 Automated Insulin Delivery System for individuals aged two years and older with type 1 diabetes.
Patrick Crannell, Senior Vice President and International General Manager of Insulet
“The addition of the Abbott FreeStyle Libre 2 Plus sensor to our CGM compatibility expands accessibility of Omnipod 5, which has been a priority for Insulet since the system launched in 2022. We are excited to have achieved this key milestone, which allows us to bring Omnipod 5 and choice of CGM to more people with diabetes.”
Tubeless Hybrid Closed Loop System
Omnipod 5 is the first and only tubeless hybrid closed loop system (also known as automated insulin delivery) that is approved for CE marking and integrated with two CGM sensor brands, Abbott FreeStyle Libre and Dexcom. The Omnipod 5 System consists of the tubeless Pod enhanced with SmartAdjust™ Technology and the Omnipod 5 Controller with its integrated Smartbolus Calculator.
The Pod’s SmartAdjust technology receives a CGM value and trend and predicts where glucose will be 60 minutes into the future. The System corrects every five minutes based on the user’s desired and customized glucose target.
Availability
Insulet expects Omnipod 5 integration with the Abbott FreeStyle Libre 2 Plus sensor to be available first in the United Kingdom and Netherlands in a phased launch in the first half of 2024, with additional markets to follow.