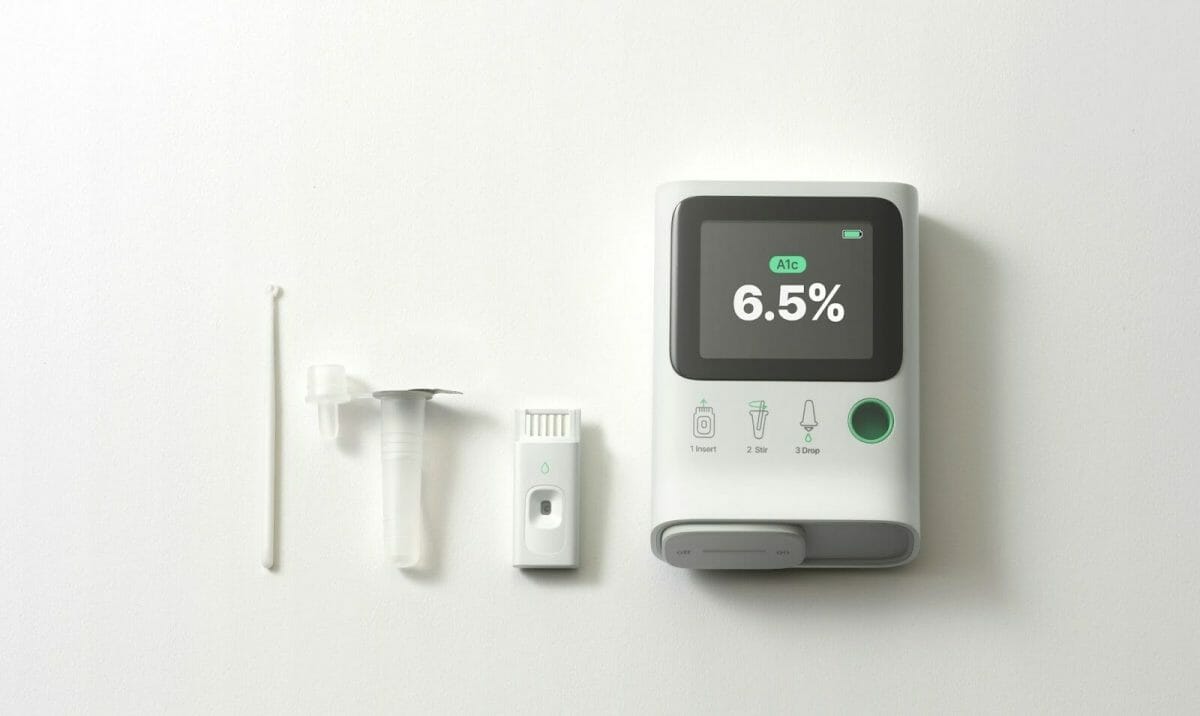
Orange Biomed—a healthcare startup with cutting-edge diabetes management technology—announced its U.S. launch today with a mission to fill critical testing gaps for people with pre-diabetes and diabetes. Orange Biomed is currently conducting studies of OBM rapid A1c, the world’s first glycated hemoglobin analyzer through single-cell analysis of red blood cells. Orange Biomed is set to unveil its latest technology at the 83rd Scientific Sessions of the American Diabetes Association (ADA), to be held in San Diego, CA, in June 2023.
“Current technologies are likely to produce inaccurate A1c results disproportionately more for non-white patients due to hemoglobin variants,” explains Co-Founder Yeaseul Park. “Hemoglobin variants are more common in patients with African, South and Southeast Asian, and Mediterranean ancestry. Inaccurate results can delay diabetes diagnosis and serious long-term health damage. We invented a new method of A1c testing, free from hemoglobin variant interference, to facilitate earlier detection and management of diabetes across all patient populations.”
Park continued, “OBM rapid A1c is a portable, guided, five-minute test requiring only a single drop of blood to produce accurate results. By seamlessly integrating lab-level technology into portable devices, we seek to make healthcare more accessible, accurate, and affordable for everyone.”
In pre-series A funding rounds, Orange Biomed has exceeded $2M in cumulative investments. OBM rapid A1c is currently undergoing global studies with Asan Medical Center. Orange Biomed is seeking FDA clearance within a year.
The latest technology will be unveiled at the ADA’s 83rd Scientific Sessions from June 24-26. Stop by presentation number 954-P in the Poster Hall to learn more. U.S. medical organizations and research centers interested in conducting trials of OBM rapid A1c are advised to reach out here.