- New Developments in Medical-Grade Microneedling Devices: Safety, Materials, and Clinical Data
- How the Right Medical Equipment Can Save your Life
- MicroTransponder Announces Positive Medicare Payment Change for Vivistim® Procedure
- Integrating NGS with Single-Cell and Spatial Platforms in Multiomic Study Designs
- Arineta Reports | Yuma Cardiology Practices Partner to Bring Advanced Imaging to Underserved Patients in Arizona with Arineta’s SpotLight™
- The Growing Importance of Vendor Partnerships in Healthcare Environments
- Smartee Denti-Technology Demonstrates World-Class Manufacturing Capacity to Support Global Growth
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
Boston Scientific receives CE mark for expanded indication of INGEVITY™+ Pacing Leads to include Left Bundle Branch Area Pacing
Conduction system pacing technique may promote greater ventricular synchrony and reduce long-term risk of heart failure associated with traditional right ventricular pacing

The Medical Device Industry | Innovations Drive Growth
Fueled by technological advances, increased demand for personalized care, and significant investment from both venture capital and corporate strategics
IVOS Medical Joins NIH’s C3i Program to Accelerate Commercialization of BOSS G4™ Airway Management Technology
Selection underscores innovation in airway safety and NIH’s support for advancing life-saving medical devices from lab to market

How To Draw Customers to Your Trade Show Booth
Steps to take to draw customers in
Clinical Trials
Valar Labs’ CHAI Biomarkers Enhance Bladder Cancer Prediction | New Study in European Urology for Predicting Outcomes of High Grade Ta Bladder Cancer
The study finds that CHAI (Computational Histology AI) biomarkers stratify recurrence and progression outcomes independently and outperform current guideline-based risk stratification
Clear Guide Medical LUMENA ™ Featured in SPIE Study on MRI-Guided Interventions
The study, titled “Augmented reality system for MRI-guided interventions: a cadaver study”, demonstrates the system’s potential to enhance MRI-guided needle procedures by improving procedural feasibility and reducing the need for repeated imaging scans.
BioArctic to Initiate Next Cohorts In Exidavnemab Phase 2a Study After Positive Safety Review
The Phase 2a study EXIST (EXIdavnemab Synucleinopathy Trial), is a randomized, double-blinded, placebo-controlled study to evaluate the safety and tolerability of exidavnemab and its pharmacokinetic profile.
Biotechnology News
Orphalan Partners with MAP International and the Wilson Disease Association to Provide Life-Changing Medicine to Underserved Communities
Through this partnership, Orphalan will donate a supply of Cuvrior® to MAP International, a global humanitarian health organization with over 70 years of experience distributing critical medicines to communities in need.
Initiation of Commercialization of ProNephro AKI (NGAL) for Diagnostic Use in the US
This purchase order is to service US hospitals through BioPorto’s distribution relationship with Roche Diagnostics.
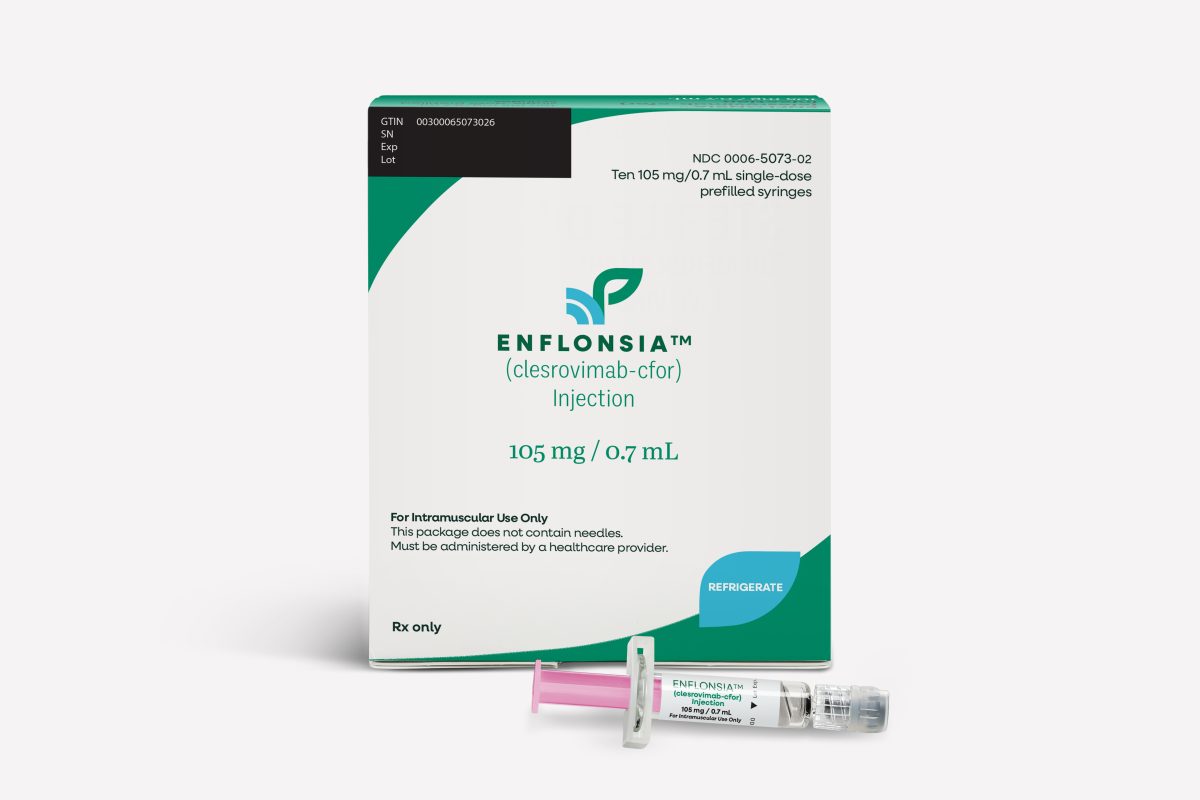
ACIP Recommends Use of Merck’s ENFLONSIA for Prevention of Respiratory Syncytial Virus Lower Respiratory Tract Disease in Infants Younger than 8 Months of Age Born During or Entering Their First RSV Season
ENFLONSIA is the first and only RSV preventive option for administration to infants using the same dose regardless of weight. Ordering will begin in July, with shipments delivered before the start of the 2025-2026 RSV season
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

Putting an End to Infusion Confusion with Smart, Simple Solutions | By Rodney W Schutt
Mr. Schutt writes: “Nurses, clinicians, and caregivers across the globe are busier and under more stress than ever before. Every day, they are expected to provide increasingly high-quality care while their resource levels remain woefully inadequate. When managing a high volume of patients, nurses face constant physical and mental strain that can quickly lead to burnout, in a space where even minor clinical errors can be harmful, even fatal.”

AI Hype vs. Reality: Medical Billing’s Need for Human Expertise | By John T Bright, CEO & Founder at Med Claims Compliance Corporation (MCC)
The U.S. healthcare system is under mounting financial pressure, driven by the abrupt rise to 55.7% in Medicare Advantage claims denials, reached between 2022 and 2023. This surge exacerbates an already costly issue: medical billing errors that drain billions annually. These errors not only strain healthcare providers but also delay reimbursements, increase administrative burdens, and ultimately affect patient care. The financial impact of denied claims and coding errors extends beyond providers; patients also suffer due to delays or unexpected out-of-pocket expenses. Compounding the challenge is a 30% shortage of medical coders, leaving organizations vulnerable to inaccuracies and backlogs in processing claims. In this complex landscape, the need for innovative, scalable solutions has never been more urgent.

Bariatric Surgery: Balancing Medications and Surgical Interventions
Discover how new weight loss medications like Ozempic are reshaping bariatric surgery practices. Dr. Monique Hassan discusses surgical innovations, treatment costs, and the future of obesity care in this comprehensive interview.
Executives on the Move

ALSAC Announces Ike Anand as President and CEO, Ushering in a New Era of Innovation and Growth
Ike Anand, who has served as interim CEO since March and as chief operating officer since 2020, steps into his new role immediately, becoming the seventh CEO in ALSAC’s 68-year history.
ONWARD Medical Adds Entrepreneur and Neurotechnology Thought Leader Tim Denison, PhD to its Board of Directors
Denison is Professor of Clinical Neurosciences at the University of Oxford and was previously Vice President of Research and Core Technologies at Medtronic. He is a co-founder and Chief Engineer of neurotechnology company Amber Therapeutics.. ONWARD Co-Founder Professor Gregoire Courtine will continue to serve as Science Advisor. He will depart the Board of Directors to address new responsibilities as Director of the Neuro-X Institute and expanded responsibilities at EPFL.
Dr. Udo Hoffmann Named Chief Medical, Scientific and AI Officer; Amit Relia Promoted to Chief Regulatory Officer at Cleerly
Strategic promotions strengthen Cleerly’s executive team as company accelerates growth.
FDA Medical Device Updates
Medline Announces FDA Clearance for REFLEX HYBRID Nitinol Implants for Foot and Ankle Surgery
“REFLEX HYBRID further demonstrates our commitment to offering innovative solutions for foot and ankle surgeons. The first to market product addresses gaps in the current competitive landscape, including offering indication specific designs, intraoperative compression, and intraoperative adjustment with a nitinol implant,” said Scott Goldstein, vice president of product management for Medline UNITE.
Terumo Neuro Receives FDA Approval for Carotid Stent System
This milestone marks the first dual-layer micromesh carotid stent approved in the United States, offering physicians a clinically proven option to improve patient outcomes in carotid artery disease treatment reports Terumo Neuro.
U.S. FDA Grants Paige Breakthrough Device Designation for AI Application that Detects Cancer Across Different Anatomic Sites
Paige PanCancer Detect recognized by FDA as a Breakthrough Device intended to assist pathologists in the detection of cancer across multiple tissue and organ types.
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
Sun Nuclear Acquires Oncospace, AI-Focused Radiation Oncology Software Provider
This strategic move underscores the Sun Nuclear commitment to leverage cutting-edge technology to improve treatment outcomes on behalf of their customers.
Oramed Pharmaceuticals Announces $36.9 Million Investment and Strategic Collaboration with Alpha Tau Medical
Oramed leverages its strategic capital markets expertise to support Alpha Tau’s breakthrough cancer therapy technology
Gestalt Diagnostics Raises $7.5 Million Series A Funding | to Expand AI-Powered Pathology Platform
The round was led by Cowles Ventures, TVF Funds, Inland Imaging Investments, KickStart Funds, and prominent angel investors from the Pacific Northwest.
Intensity Therapeutics, Inc. Announces $2.35 Million Public Offering
The Series B-1 warrants will have an exercise price of $0.85 per share, will be immediately exercisable and will expire 5 years from issuance. The Series B-2 warrants will have an exercise price of $0.85 per share, will be immediately exercisable and will expire 18 months from issuance.
Market Reports
Advancements In Imaging
Elucid Announces Fifth Medicare Administrative Contractor to Cover AI Coronary Plaque Analysis
“This latest expansion of coverage by Noridian means that even more patients could be positively impacted by PlaqueIQ, which offers the only non-invasive measurement of lipid-rich necrotic core, a vulnerable, high-risk component of plaque that can lead to heart attack and stroke,”4 said Kelly Huang, CEO of Elucid. “These recent decisions from five of the seven MACs recognize the importance of new technologies like ours that can help quantify and classify coronary artery plaque to help physicians reduce the clinical and economic burden of cardiovascular disease.”

Mediso Introduces True Theranostic TheraMAX SPECT/CT
The TheraMAX is equipped with three, large surface detectors (up to 5x larger than 12-detector CZT systems) surrounding the patients completely (360°). The 15.9mm thick NaI crystals ensure improved sensitivity for high energy photons, while the high-density sensor arrangement (123 PMT per detector head) allows high spatial resolution leading to PET-like image quality in all NM applications.

Pioneering Hospitals Take Major Steps Towards Decarbonizing Healthcare with Philips
“Good healthcare has to be environmentally sustainable, which means words and good intentions urgently need to be turned into positive actions. Philips is committed to co-creating solutions that drive measurable, impactful change. By working with like-minded action leaders around the world, sharing with them our expertise in sustainable healthcare operations, we are helping them to take critical and tangible steps toward a greener future, while at the same time enhancing patient care,” said Robert Metzke, Global Head of Sustainability at Philips.
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

How Medical Billing Software is Revolutionizing Pharmacy Operations
One area that has seen significant transformation is pharmacy operations, largely driven by the advent of medical billing software and pharmacy software systems.

Improving Hospital Culture Through Better Leadership
When leaders create an environment where people feel respected and heard, the benefits reach every corner of the hospital.

10 Tips to Expand Your Impact in Healthcare
Expanding your role doesn’t have to mean starting over or giving up your work-life balance. It can be simple, rewarding, and, yes—even fun.
Top Billing Errors in Healthcare That Can Slow Down Payments—And How to Prevent Them
The good news is that most common billing errors in healthcare are preventable with the right systems, training, and support.
Healthcare Careers

All You Need To Know About Becoming An Endoscopy Practitioner
A career in endoscopy is promising but also comes with a lot of requirements and hard work. If you are interested in becoming an endoscopy

The Future of Chiropractic Technology: Trends and Predictions
Chiropractic care in Florida is quickly transforming as new technologies are introduced. The future of chiropractic medicine looks really promising, don’t you think? With innovations

In Support of Better Medical Professional Recruitment
The recruitment of doctors is vital in ensuring high-quality healthcare. However, many hospitals and healthcare institutions have been having problems hiring and retaining physicians over
Nurses Corner
Dana-Farber Cancer Institute Nurses Have Voted to Ratify New MNA Contract | Featuring Significant Investment in Nursing and Patient Care
Registered nurses and nurse practitioners voted overwhelmingly to finalize an agreement reached in June to help DFCI recruit and retain the nurses needed to provide world-class cancer care .

How Often Should Nurses Update Their Skills?
Continuing professional development (CPD) has many advantages for nurses. Namely, being able to broaden their professional horizons while refreshing their existing skills, and also, staying abreast of industry advancements.
Laudio Expands Work With Children’s National Hospital to Include Nurse Educators
“Setting nurses up for success, supporting them effectively, and ultimately retaining them requires increasingly proactive, comprehensive strategies,” said Tom Hills, EVP of Client Engagement at Laudio. “We are excited to welcome nurse educators at Children’s National into the Laudio community.”