- The Essential Role Of Chemicals In Scientific Inquiry
- How Medical Device Distributors Accelerate Sales Growth
- Akumin Reports: First 2 Innovative Akumin AXIS™ Expandable Patient Units Deployed at Hospital Partner Locations
- Dr Jared Auclair, Co-Lead of BioConnects New England, Named 2025 Champion Public Advocate by MassBioEd
- Recor Medical Announces Paradise Ultrasound Renal Denervation System Receives Manufacturing and Marketing Approval in Japan for the Treatment of Resistant Hypertension
- Sethera Therapeutics Announces Newly Published Paper Unveils Breakthrough Enzyme That Expands Possibilities for Peptide Drug Discovery
- Spotlight Medical Announces Publication in Journal of Clinical Oncology Validating AI Pathology Assay That Identifies Low-Risk Subgroup Within Clinically High-Risk Early ER+/HER2- Breast Cancer
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
Terumo Neuro Celebrates 15 Years of the WEB Device: A Game-Changing Innovation in Aneurysm Treatment
Carsten Schroeder, President and CEO of Terumo Neuro, commented on this significant milestone: “The WEB device has set a new standard in aneurysm treatment and has been instrumental in our mission to drive game-changing impact in neurovascular care. With 15 years of proven clinical success, WEB continues to advance patient outcomes, reducing the need for multiple devices and lengthy procedures. As we celebrate this achievement, we remain committed to innovating alongside leading physicians worldwide to shape the future of neurovascular care.”
The Role of Contract Research Organizations in Modern Clinical Research
Contract research organizations assist pharmaceutical, biotechnology, and medical device companies in streamlining their operations while ensuring compliance with regulatory standards.
Smart Reactors: Revolutionizing Medical Device Coatings with Innovative Biocompatibility Solutions
Smart Reactors, an innovative medical technology company with headquarters in Ireland, is addressing this challenge head-on with its groundbreaking Camouflage™ coating technology.
Paragonix Technologies Secures Official EU MDR Approval for 5 Organ Preservation Devices
Dr. Lisa Anderson, President of Paragonix Technologies. “This achievement validates our technology’s safety and effectiveness while expanding our ability to serve patients and healthcare providers in critical transplant care across the world. With this approval and the support structure provided by Sweden-based Getinge, we are dedicated to setting new standards in organ preservation, advancing the future of transplantation, and making a global impact.”
Clinical Trials
PharmaEssentia Completes Patient Enrollment for Phase 2b EXCEED-ET Trial in Essential Thrombocythemia and Phase 3b ECLIPSE-PV Trial in Polycythemia Vera
“Myeloproliferative neoplasms, including ET and PV, are chronic blood diseases that can impact patients’ quality of life and lead to life-threatening complications, including the development of specific types of blood cancers, and PharmaEssentia is committed to developing new therapeutic solutions for these patients,” said Robert B. Geller, M.D., Head of Medical at PharmaEssentia USA. “We are thrilled with the pace at which we have enrolled patients, which we believe reflects a high level of interest in both the EXCEED-ET and ECLIPSE-PV trials. Our goal with each of these programs is to redefine the early treatment paradigm of myeloproliferative neoplasms.”
Dr Fred Cohen: Higher Temperatures Trigger Migraines According to New Study
In the study, researchers examined 71,030 daily diary records from 660 migraine patients and correlated them with regional weather data. They discovered that for each 10-degree Fahrenheit rise in daily temperature, there was a 6% increase in headache occurrences reports Dr Fred Cohen.
Micro Medical Solutions | Achieves Milestone with 200th Enrollment in HEAL Registry
reg Sullivan, CEO of Micro Medical Solutions. “This milestone is a testament to our commitment to providing innovative solutions for those suffering from CLI/CLTI. We are optimistic about the encouraging data that has emerged from the registry and look forward to utilizing this information to continue providing effective treatment options.”
Biotechnology News
EDETEK Announces New Insights on Conducting End-to-End Clinical Trial Solutions
This session, entitled, “Transforming Clinical Trials: From Reactive to Predictive,” takes place during the DPHARM Innovative Collaborations Track C, on Tuesday, September 17, 2024, at 4:10pm EDT reports Edetek.
AliveDx Receives IVDR-CE Mark for Multiplexed Assay to Accurately Detect Celiac Disease on the MosaiQ® System
“We are thrilled to obtain the CE mark for our MosaiQ AiPlex CD microarray,” said Manuel O. Mendez, CEO of AliveDx. “The MosaiQ fast portfolio expansion marks our fourth microarray CE mark approval in the last 12 months reflecting our dedication to rapidly advance diagnostic technologies and our commitment to support the millions of individuals affected by autoimmune diseases. Early and accurate diagnosis is crucial for managing this condition. Our innovative MosaiQ microarray solutions represent a significant advancement to partner with laboratorians and clinicians to reduce healthcare costs and improve patient outcomes and quality of life.”
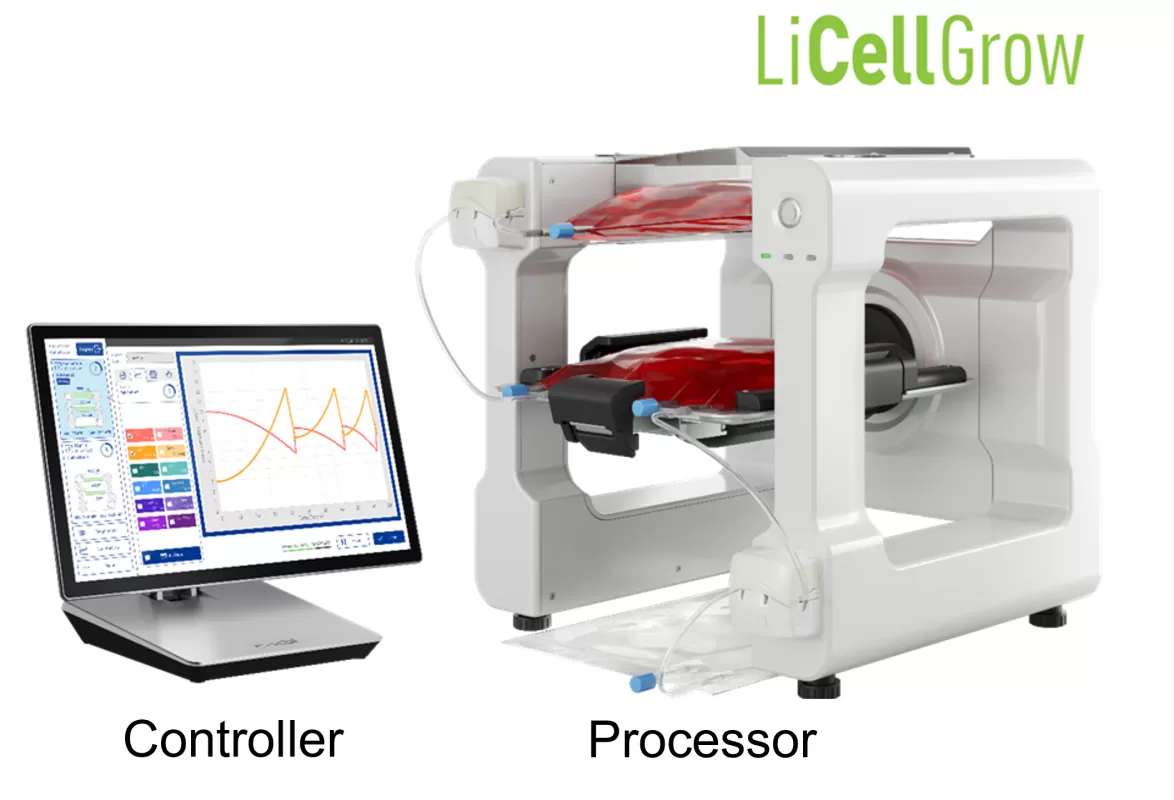
PHC Unveils Prototype of LiCellGrow, New Cell Expansion System to Support Development of Cell and Gene Therapy Products Manufacturing Process
PHC Corporation Biomedical Division (Headquarters: Chiyoda-ku, Tokyo; President: Nobuaki Nakamura, hereinafter referred to as “Biomedical Division”) will showcase a prototype of its new cell expansion system LiCellGrow at the ISCT (International Society for Cell & Gene Therapy) Europe 2024 Regional Meeting held in Gothenburg, Sweden on September 4-6.
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

Increases in Home Delivery and the Growth of Major Shippers Highlight 2024 Trends | By Tim McClatchy, Head of Life Sciences, Kenco
The growth of in-home care along with technology improvements like AI are shifting how medical device companies manage their inventory and final-mile deliveries. This, coupled by the increase of innovations like robotic-assisted surgeries, is creating a lasting impact on the medical devices supply chain. In fact, the healthcare third-party logistics (3PL) market is expected to grow at a compound annual growth rate of 7.6% from 2023 to 2030.

AI and the Human Touch: Embracing a Symbiotic Future | By Ed Watal, Founder & Principal — Intellibus
Watal writes, “Today, we see machines becoming more intelligent and capable than humans in many areas, from driving cars to diagnosing diseases. AI solves problems we cannot even approach, and it’s getting better every day — but what does this mean for us?
It’s Time to Move Beyond Mere Patient Navigation | By Craig Parker, JD, CPA and CEO, Guideway Care
Parker writes, “Is your patient navigation program delivering the outcomes you desire? Consider the following questions as you evaluate your current program. Read on.
Executives on the Move
FDA Medical Device Updates
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
Kazia Therapeutics Announces $2 Million Registered Direct Offering
Kazia has also agreed to issue in a concurrent private placement unregistered warrants to purchase up to an aggregate of 4,444,445 ADSs. The warrants will have an exercise price of $0.583 per ADS, will be immediately exercisable upon issuance, and will expire five and one-half years from the date of issuance. The closing of the offering is expected to occur on or about December 5, 2023, subject to the satisfaction of customary closing conditions.
Savage Medical Exits Stealth With Over $3 Million to Enable Minimally Invasive Colorectal Tumor Removal
The groundbreaking ColoSeal System™ enables minimally invasive colorectal tumor removal, eliminating the need for 2 open surgeries in today’s standard of care notes Savage Medical.
Alto Neuroscience Closes $45 Million in Oversubscribed Series C Financing
Alto Neuroscience advises the funds will be used to support ongoing and planned late-stage clinical development of CNS product candidates with four Phase 2 readouts expected across the next 12-18 month
Micron Biomedical Receives $23.6 Million to Accelerate Commercial Manufacturing of Needle-Free Vaccines and to Help Eradicate Measles
Micron Biomedical has received this grant from the Bill & Melinda Gates Foundation to fund mass production of needle-free vaccines utilizing Micron technology to expand the accessibility of measles-rubella vaccine in developing countries
Market Reports
Advancements In Imaging
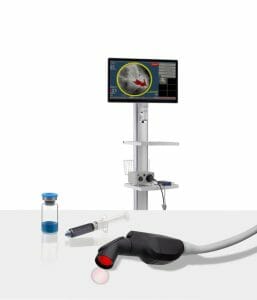
Lumicell Announces FDA Acceptance and Priority Review of New Drug Application for LUMISIGHT™ Optical Imaging Agent for Breast Cancer
The Lumicell DVS is an investigational system designed for use in patients with breast cancer to assist in the detection of residual cancerous tissue within the lumpectomy cavity following removal of the primary specimen during breast conserving surgery.
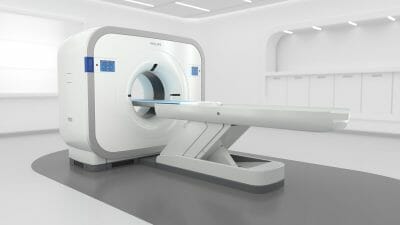
AI-powered CT System Launches Reports Philips
Philips CT 3500, is a new high-throughput CT system targeting the needs of routine radiology and high-volume screening programs. Powered by AI, the Philips CT 3500 includes a range of image-reconstruction and workflow-enhancing features that help to deliver the consistency, speed, and first-time-right image quality needed for confident diagnoses by clinicians and increased return on investment – even in the most demanding, high-volume care settings.
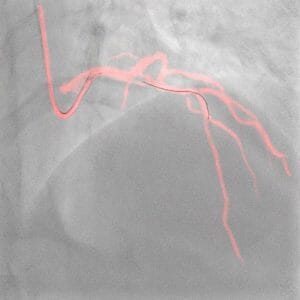
Philips’ Image-guided Navigation Increases Safety During Coronary Interventions and Reduces the Use of Contrast Media by an Average of 28.8%
The results were presented today at the European Association of Percutaneous Cardiovascular Interventions (EuroPCR) 2023 course and will also be presented later this week at the Society for Cardiovascular Angiography & Interventions (SCAI) 2023 scientific sessions.
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

What Are the Top Skills Every Future Doctor Needs to Perfect?
From the delicate art of suturing to mastering advanced communication, in this article we’ll explore the multifaceted world of modern medicine.

How Can Online Exam Software Improve CPR Training and Certification?
As more and more human beings opt for guides on CPR education, the need for this education becomes increasingly critical.

Systematic Education for Sedation: A Guide for Hospital Leaders
This guide provides comprehensive recommendations for implementing effective systematic education for sedation for healthcare professionals. Read on to learn more.

The Importance of Employee Development Plans for Your Company and Staff
In today’s business environment, it has become crucial for organizations to prioritize employee development plans as a means to foster growth and drive success. It’s
Healthcare Careers
Nurses Corner

9 Tips For Critical Care Nurses
Nurses today are worried about being overworked, understaffed, and seriously exhausted. Among many nursing vocations, critical care nurses have more duties than traditional RNs. Experts

How to Find the Best Nursing Services?
Very often it is difficult to get the desired care at home. In most cases, these services are limited and do not always correspond to

8 Advantages of Choosing a Nursing Career
In this digitized world, people are becoming more career-oriented and goal-driven. People no longer want to work for just a paycheck; they want jobs that




