- FDA Grants De Novo Clearance for Reflow Medical’s Spur® Peripheral Retrievable Stent System
- GC Biopharma’s Phase 3 Clinical Trial Results for Hunterase Published in SCIE-Indexed Journal
- Bionical Emas and Pharma Resources Announce Exclusive Global Clinical Trial Supply Partnership
- Isotopia and Cardirad Announce Launch of Isoprotrace ® in Sweden and Denmark, Expanding Access to Advanced Prostate Cancer Imaging
- Precision Medicine – Closing the Loop: Why a Test Alone Isn’t Enough | By Dr Tom Stubbs – CEO Hurdle
- MellingMedical Adds the joimax® Suite of Full-Endoscopic, Minimally Invasive Spinal Surgery Products to Its Federal Health Portfolio
- Olympus Announces Launch of New Endoscope Drying Cabinet
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
Baird Medical Devices Granted New Class III Certificate by China’s NMPA for Ceramic Thyroid Ablation Needle
“The introduction of the Ceramic Thyroid Ablation Needle signifies another breakthrough innovation in the field of microwave ablation and is a testament to our ongoing commitment to innovation and research,” said Ms. Haimei Wu, Founder and CEO of Baird Medical Devices. “We eagerly anticipate the positive impact of this technology by enhancing the patient treatment experiences and saving lives in the years to come.”
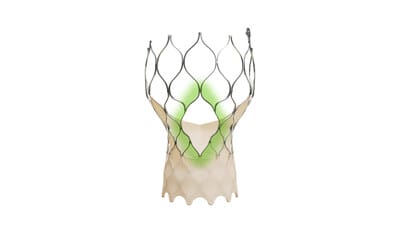
Medtronic Announces FDA Approval of Newest-generation Evolut TAVR System for Treatment of Symptomatic Severe Aortic Stenosis
The Evolut ™ FX+ TAVR system leverages market-leading valve performance with addition of larger windows to facilitate coronary access
Eagle Telemedicine Announces Eagle MedWorks Connect Modular Telemedicine Cart System
Jason Povio, CEO, Eagle Telemedicine, “Increasing access to care through telemedicine shouldn’t be constrained by cost and technology implementation barriers. To remove these obstacles, we have developed the Eagle MedWorks Connect modular telemedicine cart. Built, end-to-end, for clinical ease of use and seamless integration with existing hospital technology, the new cart solution is not only cost-effective to procure but even easier to implement, manage, and use. It is an integral part of Eagle’s suite of solutions to improve access to care, anywhere.”

World’s 1st Total Knee Replacement Patient Treated with PolarisAR Breakthrough Mixed-Reality Surgical Guidance System
PolarisAR describes STELLAR Knee as a mixed-reality surgical navigation platform that guides TKA procedures by displaying measured and computed data overlaid directly in a 3D environment. The mixed-reality system acts as a spatial computer, creating continuous data exchange between the surgeon and the software to help enhance surgical decision-making while simplifying operating room workflow.
Clinical Trials
Antengene Presents Encouraging Clinical Data from Four Pipeline Programs at the 2023 R&D Day
Antengene notes Clinical programs, including ATG-101 (PD-L1/4-1BB bispecific antibody), ATG-022 (Claudin 18.2 antibody-drug conjugate), and ATG-037 (oral CD73 inhibitor) have shown clinical responses in cancer patients with advanced diseases. And ATG-008 (dual mTORC1/2 inhibitor) continued showing strong clinical activities in the Phase II program, with promising efficacy data in cervical cancer patients compared to benchmarks.
Surmodics Announces 24-Month Data from the SWING Trial Presented at VEITHsymposium
The SWING Trial is a 35-subject prospective, multi-center, single-arm, feasibility study to evaluate the safety and performance of the Sundance Sirolimus DCB when used to treat occlusive disease of the infrapopliteal arteries. The SWING Trial enrolled subjects with stenotic or occluded lesions of the infrapopliteal arteries, a reference vessel diameter of 2 mm to 4 mm, and a total lesion length of ≤230 mm for treatment with the Sundance Sirolimus DCB at eight sites in Australia, New Zealand, and/or Europe. Study subjects will be followed for 36 months post index procedure.
Surmodics Announces SWING Trial 24-Month Data to be Presented at VEITHsymposium on November 15
Professor Varcoe, MBBS, MS, FRACS, PHD, MMed (ClinEpi), co-lead investigator of the SWING Trial, is a vascular surgeon at Sydney’s Prince of Wales and Prince of Wales Hospital where he is Director of Operating Theatres, and Director of Surgery and Anesthetics for the South East Sydney Health District. He will review safety and performance data collected through 24 months of follow-up for 35 subjects with occlusive disease of the infra-popliteal arteries who were treated at study sites in Australia, New Zealand, and locations in Europe. Study subjects will be followed for 36 months after the index procedure notes Surmodics.
Biotechnology News
PacBio Announces HiFi Prep Kit 96 and HiFi Plex Prep Kit 96 for Long-Read Sequencing Applications at Scale
The new HiFi library prep kits are expected to be available for purchase in the first quarter of 2024, with delivery planned early in the second quarter of 2024. Automated protocols will first be available on the Hamilton NGS STAR system, with qualified protocols on additional PacBio Compatible automation platform partners being added in the future. Kits contain enough volume for customers to develop their own automated methods notes PacBio.

Effortless Measurement of All Four NADs and Glutathione for Laboratories
“We believe that the NADMED method will advance science and medical practice by enabling larger and quicker clinical trials and in due course, diagnostics. NAD measuring should be seen as a first-line diagnostic tool in the future to fight for example degenerative diseases and metabolic disorders.”
Lonza and Oxford Nanopore to Collaborate on Novel Test to Accelerate Analysis of mRNA Products
The collaboration aims to cGMP validate and commercialize a first-of-its-kind novel test to accurately determine multiple critical quality attributes of mRNA products by directly sequencing both the DNA template and the messenger RNA (mRNA).
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

A Revolution in Cardiovascular Care By Seth Bogner, Chairman, and CEO of HeartPoint Global
Mr. Bogner writes, “While the challenge of treating cardiovascular disease is daunting, I am optimistic about the progress we have made by developing a revolutionary MedTech device that will provide a solution for patients with privilege and without. I am looking forward to the next milestone of our first human use trials in Europe in early 2023.” Read more what he has to say.

Medical Grade Voice Biomarker AI Will Transform Early Disease Diagnoses By: Tamir Tal, CEO, Cordio Medical
The potential of voice biomarkers is advancing and opening new opportunities for diagnoses, risk prediction, clinical outcomes, and symptomology. As one of the most promising digital healthcare sectors, all age cohorts, especially the aging population, may see the most benefit. Read what Mr. Tal has to say.

Vertebrogenic Pain: New Society Guidelines Highlight Treatment for this Distinct Type of Chronic Low Back Pain By Ray Baker, MD, Chief Medical Officer, Relievant Medsystems
In the October 2022 issue of the International Journal of Spine Surgery, the International Society for the Advancement of Spine Surgery (ISASS) published an updated Policy Statement and Literature Review of Intraosseous Basivertebral Nerve Ablation. This revised statement followed the September 2022 publication of Best Practice Guidelines on the Diagnosis and Treatment of Vertebrogenic Low Back Pain with Basivertebral Nerve Ablation from the American Society of Pain and Neuroscience (ASPN).
Executives on the Move
FDA Medical Device Updates
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
Lauxera Capital Partners Invests €10m In Matrix Requirements
Founded in 2014, Matrix Requirements supports the R&D, Regulatory Affairs and Quality departments of MedTech companies by providing specialized digital tools for medical device design, traceability, quality management and regulatory compliance.

Able Innovations Works to Transform Frontline Healthcare with USD $6MM in Funding
Able Innovations is using robotics to transform processes for healthcare staff by incorporating safety and dignity into a routine procedure that affects providers and patients.
PathMaker Neurosystems Receives $100,000 START Award from MassVentures
PathMaker Neurosystems notes this Stage I START funding was awarded on the basis of PathMaker’s $4.9M grant from the NINDS Cooperative Research to Enable and Advance Translational Enterprises for Devices (CREATE Devices) program to develop MyoRegulator®, a first-in-class, non-invasive neuromodulation device for treating muscle spasticity.
QuidelOrtho Formed by the Completion of Transaction Combining Quidel and Ortho Clinical Diagnostics
The new company, headquartered in San Diego, California, generated more than $3.5 billion in combined revenues in 2021 and has approximately 6,000 employees. QuidelOrtho will trade on the Nasdaq Global Select Market under the symbol “QDEL.”
Market Reports
Advancements In Imaging

Engenious Design Partners with Precision Microwave
Engenious Design advises system is a new Microwave Generator to accompany Precision Microwave’s next-generation Directional Microwave Ablation applicator.
AMRA Medical Advises MRI-Based Muscle Assessment Technology Approved for Clinical Use in US and Canada
AMRA Medical is now permitted to distribute AMRA® BCP Scan (Body Composition Profile) in the US and Canada.

Tongji Hospital in China to Equip New Proton Therapy Center with MEVION S250i Proton Therapy System®
Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology to equip their new proton therapy center with a MEVION S250i Proton Therapy
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

Six Ways to Improve Patient Experience in Hospitals
A healthcare institution’s success is determined by patient satisfaction and personalized visits. A recent Deloitte-Scottsdale Institute survey on digital transformation initiatives in health systems showed

The Key Benefits of Using a LIS (Lob Information System) in Healthcare
Laboratories worldwide are battling with astronomical amounts of data and constantly looking for efficient ways to manage it. Old-fashioned laboratories no longer work because of

Improve Client Satisfaction In Your Medical Spa With These Strategies
Every business needs to build a solid customer base because it’s the lifeblood of any organization, and medical spas are no different. Ensuring your customers

QR Codes In Medical Device Marketing – Use Them Everywhere
QR Codes are a quick, simple, & a touch-free way for medical device companies to direct customers to online content. Learn why and how they can benefit you in your medical device marketing. Read more.





