- Hyperfine Announces FDA Clearance of Optive AI™ Software, Introducing the Most Substantial Image Quality Improvement Ever for the Swoop® System
- AVNA INC. Appoints David Bonvenuto and Aaron Mambrino to Board of Directors
- Octapharma Releases Results for Phase 3 Superiority Study of Pediatric Acute-onset Neuropsychiatric Syndrome
- Pimicotinib Demonstrates Best-in-Class Potential with Significant Efficacy and Clinically Meaningful Improvements in Patients with Tenosynovial Giant Cell Tumor
- BD to Initiate Real-World Patient Data Registry for the Rotarex™ Atherectomy System in Treatment of Peripheral Artery Disease
- £48 Million Investment for World-leading Wound Innovation Centre
- New Drug Approval for In-House Developed Anti-Insomnia Drug DAYVIGO (Lemborexant) in China
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook

IDC Partners With XFT Medical to Create an Innovative Hand Rehabilitation Device
The rehabilitation robotic glove is a training device that combines EMG electromyographic feedback and flexible robotics for patients with stroke or hand disabilities to train and repair dexterity.
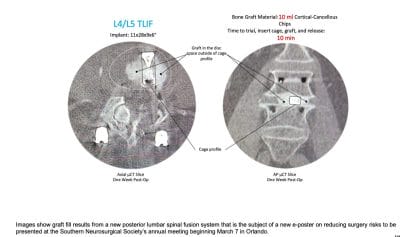
Kleiner Device Labs Presents e-Poster at Southern Neurosurgical Society Meeting
Kleiner Device Labs will attend the meeting and looks forward to demonstrating the new KG®2 Surge® flow-thru interbody system to surgeons
Medtronic Files ITC Action Against Axonics to Stop Unauthorized Use of Medtronic Innovations
“Medtronic is continuing our efforts to stop Axonics from profiting off of their unauthorized use of our innovations and intellectual property,” said Mira Sahney, president of the pelvic health business in the neuroscience portfolio at Medtronic. “The pattern is clear: Axonics uses Medtronic technologies to improperly compete in the market. It is time for Axonics to be held accountable for these unlawful acts.”
Manufacturer of Industrial Telescopic Slides and Linear Guide Rails
Industrial telescopic slides and linear guide rails are used in various applications, including manufacturing equipment, machine tooling, and industrial automation. Read on to learn more.
Clinical Trials
Alimentiv, AcelaBio, and PharmaNest Unite To Revolutionize Precision Medicine and AI Digital Pathology For NASH/MASH Clinical Trials
This collaboration will enable clinical trial sponsors to quantify the histological effects of compounds and gain deeper insight into underlying mechanisms in MASH-targeted therapies using state-of-the-art spatial transcriptomics and AI-powered single-fiber and single-cell digital pathology.
Surmodics Announces TRANSCEND Trial 36-Month Data to be Presented at 50th Annual VEITH Symposium
Dr. Peter A. Schneider, Professor of Surgery at University of California, San Francisco and Member of the TRANSCEND Trial Steering Committee, will present the TRANSCEND trial’s safety and efficacy data through 36 months as part of a November 15, 2023 session focused on randomized controlled trial (RCT) updates on new developments in drug coated balloons (DCBs) and drug eluting stents (DESs).
NICO Corporation: Study Shows First-Ever Successful Deep-Brain Implant of Computer Chip in Living Animal
NICO Corporation notes the proof-of-concept study was published in Brain-Computer Interfaces in September and used a modified and miniaturized NICO BrainPath, the world’s first navigated trans-sulcal access technology widely used in Minimally Invasive Parafascicular Surgery (MIPS) for access in the removal of brain tumors and evacuation of hemorrhagic stroke.
Biotechnology News
BIOTRONIK’s DX Technology Reaches Significant Milestone: 100,000 DX Devices Implanted
BIOTRONIK notes Over the past decades, more than 20 clinical studies that enrolled over 4,000 patients have confirmed the safety and efficacy of DX Technology. This strong foundation of clinical evidence has recently been enriched by the MATRIX study results, the largest clinical evaluation of DX Technology to date. Results from the MATRIX study confirm under real-world conditions that DX, with Home Monitoring, allows for reliable, guideline-recommended remote monitoring of subclinical AF.
AtlasXomics and EpiCypher Partnership | To Commercialize Spatial Epigenomics Assays
AtlasXomics and EpiCypher have focused initial development efforts on fresh frozen samples. Their long-term goal is to optimize spatial CUT&Tag for formalin-fixed paraffin-embedded (FFPE) samples, thereby greatly enhancing the versatility of these assays for clinical applications.

BioConnects New England (BCNE) Partners with Thermo Fisher, Mass Life Sciences Center and Northeastern University to Host Booth at JA Inspire
BioConnects New England (BCNE) believes that the future of biotech relies heavily on creating access, education, and connection to the industry for young people. Junior Achievement Boston has built a brilliant opportunity with its annual JA Inspire Event.
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

MedTech Trends for 2023 By Bernard Ross, CEO and Founder of Sky Medical Technology
Healthcare services have been buffeted on the rocks of the global pandemic since early 2020, and many are now trying to address the significant backlog in non-urgent and elective surgeries that have been postponed by primary healthcare focusing on COVID-19.
As we enter the post-pandemic era, Bernard Ross, CEO, and Founder of Sky Medical Technology outlines his views on the direction of travel of the healthcare industry in 2023 and the implications for MedTech.

Defining Care Pathways in a Post-Stroke World – By David Z. Rose, M.D.
Dr. David Z. Rose is a Vascular Stroke Neurologist at the University of South Florida College of Medicine in Tampa, Florida, USA, and Co-Medical Director of the 32-bed Neuro-ICU at Tampa General Hospital. He is the author of a fun review book entitled “Laughing Your Way to Passing the Neurology Boards” and many peer-reviewed articles on Neuro-Cardio topics. He completed an Internal Medicine internship and residency at the Cleveland Clinic in Ohio, Neurology residency at the University of Miami/Jackson.

The Evolution of OTC vs. Prescription Wearable ECG Monitoring Devices – Differences and Considerations for Use: By Ann Demaree, MBA, BSN, RN, Senior Vice President, Cardiac Insight LLC
Ann Demaree writes, “While OTC wearable devices are designed to allow for augmented individual monitoring of body parameters, identifying deviations that can empower people to make educated decisions on their personal fitness and health, the question is, just how accurate are they and where should they fit within the ideal healthcare ‘ecosystem’?”
Executives on the Move
FDA Medical Device Updates
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
Synergy Health Network’s $100 Mil Expansion Starts with Crowdfunding via Rialto Markets
Synergy Health Network is giving investors the chance to back its expansion plans after teaming up with Rialto Markets to utilise the award-winning broker-dealer’s crowdfunding platform and infrastructure for a $1 million Reg CF raise to launch Synergy’s four-year growth mission.
Deciphex Raises $11.5M in Series B Funding Led by ACT Venture Capital
Deciphex will leverage the funding to continue driving global growth and to consolidate its position as a leader in revolutionising clinical and non-clinical pathology
Project for Futuristic Surgical Training Receives CHF 12 Million in Funding
Surgical training: “With the ‘PROFICIENCY’ project, we want to advance simulator-based continuing education in surgery,” explains Prof. Bruno Schmied, M.D., Chief of Surgery at the Cantonal Hospital of St. Gallen.
Marquee Tech Founders & Investors Join Ultrahuman’s Series A
Ultrahuman, a leading metabolic fitness platform that uses glucose biomarkers to help people optimize their lifestyle.
Market Reports
Advancements In Imaging
KA Imaging Secures Taiwan License for Reveal 35C Dual-Energy X-ray Detector
Reveal 35C is the world’s first and only single exposure dual-energy flat panel X-ray detector that can be used in fixed, mobile, and portable applications.
Compact Proton Therapy System by Hitachi at Shonan Kamakura Advanced Medical Center
Until now, there have been no proton therapy facilities in Kanagawa Prefecture, where the center is located, but the compact proton therapy system, with its reduced footprint, makes it possible to install a proton therapy facility on a limited site close to the city center.
Carestream Continues Drive to Improve Image Quality and Workflow With 45 Patents in 2021
“Carestream continuously strives to deliver cutting-edge technologies worldwide, and these patents demonstrate our commitment to meet the evolving needs of providers and patients alike,” said Eugene Shkurko, Intellectual Property Counsel at Carestream.
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

How Can Offering Telehealth Help My Patients?
One of the best ways to keep up with modern trends in medicine is to offer telehealth to your clients. If you are a medical

The Importance of Sterilization Equipment in Ensuring Workplace Safety
Maintaining workplace safety is crucial, and sterilization equipment plays a vital role in achieving this goal. This article explores the significance of sterilization equipment in
Tips for Reporting Clinical Studies in News and Public Media
As a news or media reporter, you should follow some guidelines in order to prevent misinformation and legal problems.

The Importance of Digitalization in Healthcare
Digitalization in Healthcare: The importance of the NHS as an institution need not be stated. It is one of the UK’s most popular and highly-regarded




