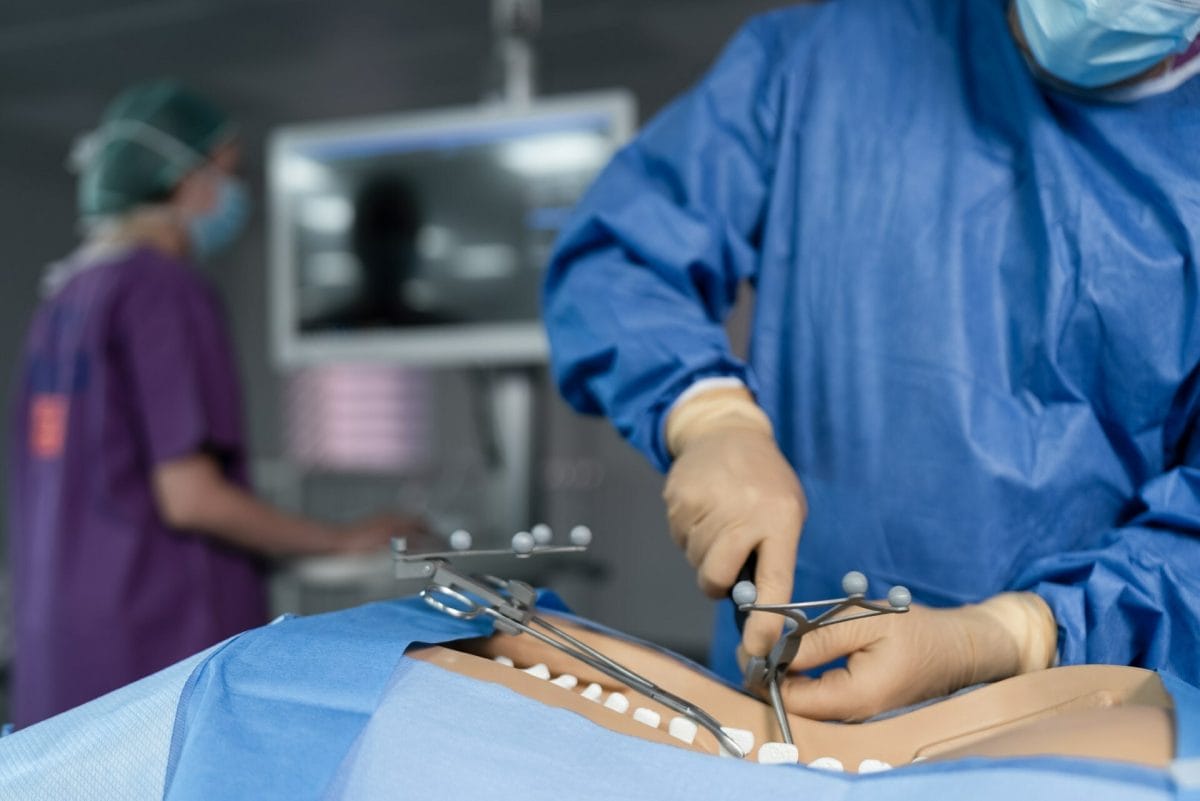
PathKeeper Surgical, a privately-held, Israel-based, medical technology company, dedicated to improving the health of individuals around the world suffering from all spinal issues requiring surgery. While celebrating the first use of the PathKeeper 3D optical navigation system during a spinal fusion surgery at Southcoast Health’s St. Luke’s Hospital in New Bedford, Massachusetts, PathKeeper Surgical strives to make navigation-guided surgery available to any patient in all operating rooms.
The PathKeeper system was utilized to create a comprehensive surgical plan and accurately navigate the single-level, lumbar degenerative spinal fusion surgery at Southcoast Health’s St. Luke’s Hospital.
“The PathKeeper system addresses many of the intricacies of spine surgery and adjusts to individual patient needs with a high level of accuracy and no radiation during a crucial part of the surgical procedure,” said Matthew Philips, MD, Chief of Brain & Spine Services, Southcoast Health Neurosurgery, Dartmouth, MA. “We have already experienced the clinical benefits of incorporating the PathKeeper system into our spinal fusion surgeries at St. Luke’s Hospital.”
The PathKeeper system was designed to replace traditional navigation technology with a 3D optical navigation system that offers comprehensive surgical planning; active, independent, submillimeter registration and tracking of the patient anatomy and surgical instruments; pinpoint accuracy of device implantation; more efficient surgical workflow; elimination of radiation exposure during the surgical procedure; and a more economical price so both the hospital and ambulatory-surgical center operating rooms can incorporate this new technology.
“We are thrilled at the adoption of the PathKeeper system at Southcoast Health, which continues our expansion of the PathKeeper system to hospitals and surgery centers across the United States,” said Ryan LeBlanc, Chief Commercial Officer, PathKeeper Surgical. “The PathKeeper system is a game changer in driving improved clinical outcomes and economic value to patients and healthcare providers.”
PathKeeper Surgical received its FDA 510k clearance earlier this year for the PathKeeper system. The name PathKeeper effectively describes the essence of the system – a 3D optical navigation system that ‘keeps’ the surgical ‘path’ on course throughout the surgery.