PathKeeper Surgical has been selected to exhibit their Artificial Intelligence (AI) and Optical Spine Navigation solution at the Vizient Innovative Technology Exchange. Vizient, Inc., the nation’s largest provider-driven healthcare performance improvement company, will hold the Exchange Sept. 18 in Las Vegas.
The annual Innovative Technology Exchange offers selected suppliers the unique opportunity to demonstrate their product or service to supply chain and clinical leaders from Vizient’s customer hospitals and subject matter experts who serve on their supply councils. Each product or service will showcase how it improves clinical outcomes, enhances safety or drives incremental improvements to health care delivery or business models.
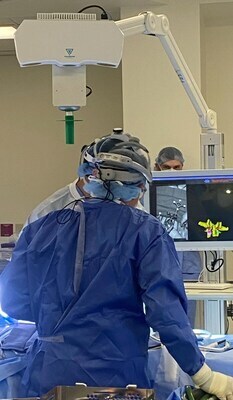
PathKeeper Surgical is working to revolutionize Spinal Navigation with AI and IR Laser Optics. These combine to create a digital surgical platform, with no intraoperative radiation and accuracy for registration and tool tracking to less than a millimeter. Furthermore, it enhances surgical workflows, greatly reduces radiation to patients, and delivers a solution that can help across multiple settings including the Ambulatory Surgery Center as well as for Deformity Surgeons in hospitals.
“We are very excited to bring PathKeeper to the Vizient Innovative Technology Exchange,” said Erez Lampert, CEO of PathKeeper. “Our mission is to revolutionize the spine surgical landscape by integrating advanced technologies that prioritize patient safety and surgical precision. Our advancement significantly enhances surgical workflows and reduces radiation exposure, benefiting both patients and medical professionals. We are excited to bring our innovative solution to market with Vizient, where it can make a real difference in diverse surgical settings, democratizing technology for spine surgeons and patients everywhere.”
“The selection process for medical device and supply companies that exhibit at the Exchange is a rigorous one,” said Kelly Flaharty, senior director of contract services, Vizient. “Companies showcase their products and services hoping to be awarded an Innovative Technology contract — a signal to healthcare providers of their solution’s unique qualities.”
The annual Innovative Technology Exchange is part of Vizient’s Innovative Technology Program that includes product review of supplier-submitted technologies by provider-led councils. Since 2003, Vizient has reviewed over 1,600 product submissions as part of its Innovative Technology Program.