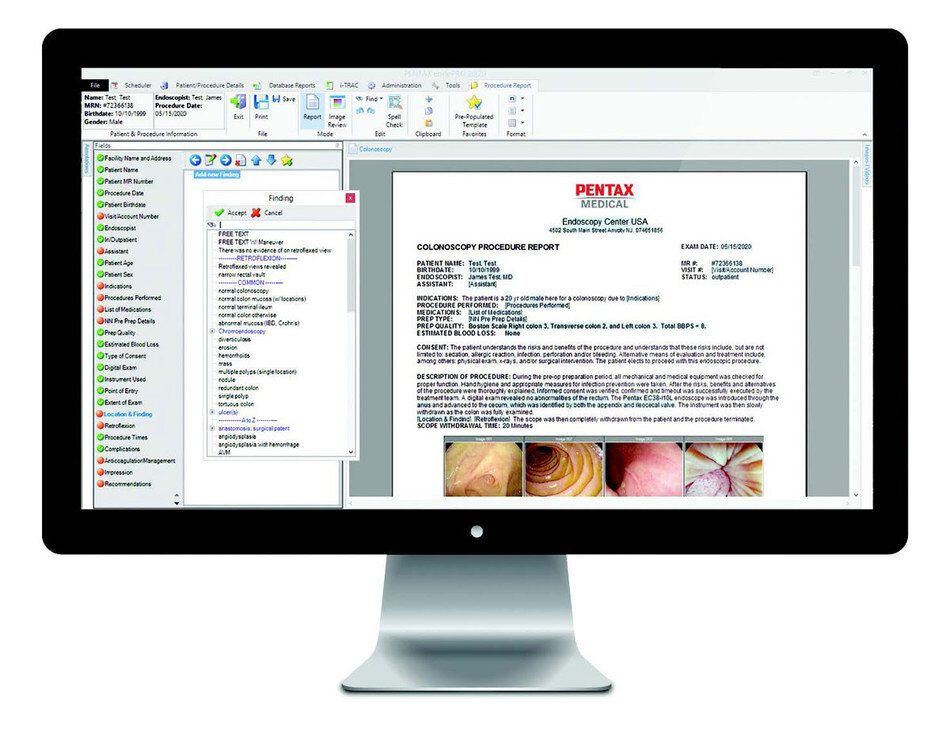
PENTAX Medical announced today the launch of the new endoPRO 20|20 software, a distinctive endoscopy image management and patient data analysis system that provides efficiency-focused workflow enhancements and ensures pathology results are available in a streamlined and timely manner.
PENTAX Medical notes their next-generation system, the endoPRO 20|20 pathology engine offers functionality that leverages HL7 Interfacing and PENTAX Connect to create interoperability between disparate hospital systems, delivering efficient procedure reporting and quality indicators.
With diminishing reimbursement and growing pressure on physicians to increase volume, capturing and reporting appropriate quality indicators to increase operational efficiency and to facilitate clinical improvement has become imperative for facilities and practitioners. The new endoPRO 20|20 software has been specifically developed to fulfill these needs, with a focus on providing a seamless pathology workflow, integration, and automation.
EndoPRO 20|20 offers a comprehensive system to support efficiency at each stage of the workflow, from requisition to results to patient follow-up, also providing specific options to best suit facility needs and processes. Among the new features, it aids physicians in the calculation of Adenoma Detection Rates (ADR), transforming a resource-intensive process into one that is automated and efficient, so endoscopists no longer need to calculate their ADR manually. Additionally, endoPRO 20|20 eliminates the need for pathology results to be siloed in the Electronic Medical Record (EMR) or paper patient records, allowing the physician to review the pathology while they are completing procedure reports.
Further key highlights of the endoPRO 20|20 pathology engine include the ability to generate notifications for physicians when new results are available. The engine will also analyze a patient’s pathology results based on specific categories and recommend appropriate follow-up examination dates based on those results and endoscopy society guidelines.
With endoPRO 20|20, there is also the addition of DICOM support, which facilitates the easy transmission of procedure images to the hospital PACS system for retrieval or printing, as well as compatibility with the recently introduced PENTAX Connect for Capsule Endoscopy that interfaces the CapsoCam Plus software with the hospital EMR software.
“As an industry leader, PENTAX Medical is proud to combine the best in endoscopic equipment, software solutions, and services, all in a single turn-key operation. Our team holistically focuses on helping our customers reach their Triple Aim objectives—increase efficiency, decrease healthcare costs, and most importantly, advance patient care,” said Rainer Burkard, Chief Commercial Officer at PENTAX Medical, Americas, “We know our customers value our commitment to the informatics space and as such will be very pleased with the new functionality offered by endoPRO 20|20.”
Technology advancements are rapidly creating opportunity for improvement in endoscopy treatment. Standardized treatment protocols and software application interfacing are changing the way patient care is provided. With its focus on innovation, and systems such as endoPRO 20|20, PENTAX Medical is paving the way for hospital performance in endoscopy.