March 2, 2021
Philips and DiA Imaging Analysis Ltd., a leading provider of AI-powered applications for ultrasound, today announced a strategic partnership* to deliver automated solutions to clinicians at the point-of-care.
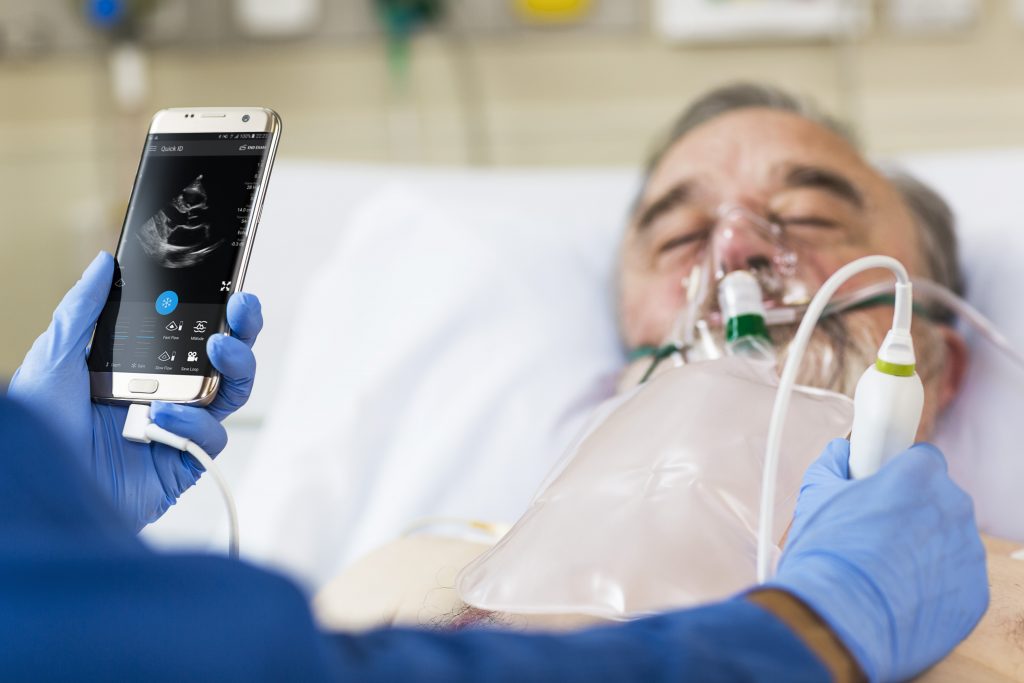
Philips’ industry leading ultrasound image quality combined with DiA’s AI library of automated solutions will help increase diagnostic confidence, operational efficiency, and access to care for Point-of-Care customers in and out of the hospital.
Philips continues to build suites of intelligent and automated enhancements integrated into Radiology workflows across its portfolio to benefit customers and patients alike. By facilitating the development and integration of AI-enabled applications, Philips aims to enhance its ability to deliver on the Quadruple Aim of better patient outcomes, improved patient and staff experiences and lower cost of care. The integration of AI applications within ultrasound technology can also help lead to improved access to care.
While ultrasound is a cost-efficient diagnostic imaging modality, it currently requires a significant level of operator experience and visual interpretation to use it effectively. In particular, the subjectivity and variability associated with visual interpretation pose a challenge for longitudinal assessments to gauge patient improvement or deterioration. To reduce the variability, improve the efficiency and increase the accuracy of image analysis, DiA Imaging has developed FDA cleared and CE-marked AI-enabled 2D ultrasound applications.
“This partnership with DiA Imaging Analysis reinforces Philips’ commitment to integrating AI partners into the Radiology workflow ecosystem, from the modality, to interpretation, all the way through to results communication,” said Kees Wesdorp, Chief Business Leader, Precision Diagnosis at Philips. “Through our strategic partnership with DiA Imaging Analysis, we can deliver unprecedented diagnostic confidence and operational efficiency to point-of-care ultrasound imaging, as well as widen access to high quality and timely care both inside and outside of hospitals.”
“With superb image quality and ability to operate with smartphones and tablets, Philips Ultrasound devices are widely accepted as the industry’s most advanced solution for point-of-care imaging. As such, it is the ideal platform for deploying AI-enabled solutions that will benefit patients by helping clinicians to make better-informed decisions,” said Hila Goldman Aslan, CEO and Co-Founder, of DiA Imaging Analysis Ltd.
DiA’s AI technology automates the process of manually capturing and visually analyzing ultrasound images. The company’s LVivo Toolbox includes a range of FDA cleared and CE marked automated AI-enabled apps focused on cardiac and abdominal ultrasound analysis to help support clinicians with varying levels of ultrasound experience.
Philips will spotlight its AI-enabled solutions across its imaging portfolio at the upcoming European Congress of Radiology 2021 virtual event (March 3-7, 2021). For more information on Philips’ new portfolio of diagnostic and interventional solutions, including the new Philips Abdominal Aortic Aneurysm (AAA) Model which seamlessly integrates 3D Ultrasound with innovative software in a single solution, visit the Philips ECR site, and follow @PhilipsLiveFrom for updates throughout the event.
* As part of the partnership, Philips owns a minority interest in DiA Imaging Analysis.