Pie Medical Imaging (“PMI”), (cardiac imaging), part of the Esaote Group, today announced the beginning of FASTIII, a multicenter randomized clinical trial that will investigate the use of angiography-based vessel fractional flow reserve (CAAS vFFR) in patients undergoing coronary revascularization procedures.
The FASTIII trial, that is led by Dr. Joost Daemen (cardiologist at the Thoraxcenter at the Erasmus University Medical Center, Rotterdam, The Netherlands), is an investigator initiated international, multi-center randomized, non-inferiority trial aiming to enroll a total of 2228 patients, in 7 European countries and 35 hospitals and is sponsored by the European Cardiovascular Research Institute (ECRI).
The FAST III study aims to demonstrate non-inferiority of CAAS vFFR guided revascularization as compared to a conventional invasive wire based FFR guided revascularization in patients with either stable coronary syndrome or non-ST segment elevation myocardial infarction and intermediate coronary artery lesions.
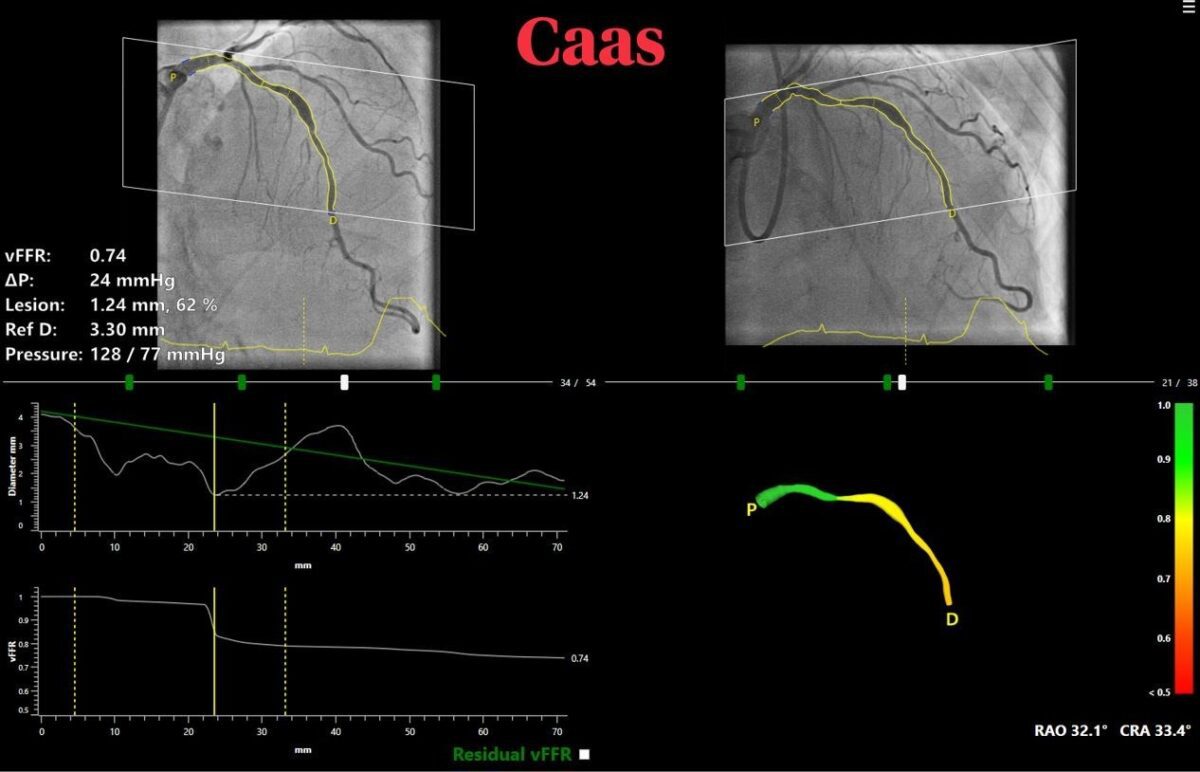
vFFR can assess whether a coronary artery narrowing is functionally severe and requires treatment. CAAS vFFR allows doing so without the need of invasive wires- that are part of the routine practice to measure pressure gradients (FFR) – and adenosine.
The high diagnostic accuracy of CAAS vFFR, which calculates pressure drop and vFFR value using angiography images only was recently confirmed by the results of FAST I, FAST Extend and FASTII studies which validated vFFR as an accurate and easy to use tool to assess coronary physiology.
“We are confident that this new study will lead to a broader use of methods based on angiographic images for a safe and accurate assessment of severity and percentage of artery stenosis” said René Guillaume, PMI Managing Director.
The trial is funded by research grants from Pie Medical Imaging (Maastricht, the Netherlands) and Siemens Healthineers GmbH (Erlangen, Germany).