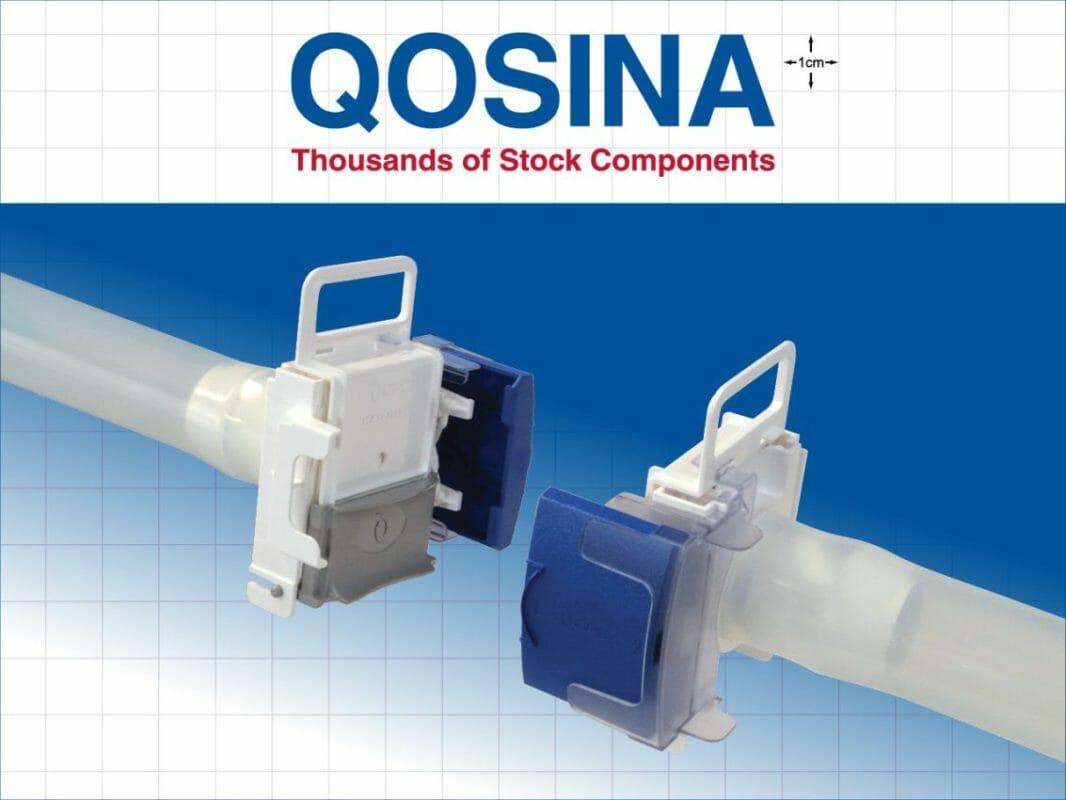
Qosina, a global supplier of OEM single-use components to the medical and biopharmaceutical industries, is pleased to introduce CPC’s (Colder Products Company) new AseptiQuik® W Series connectors to its extensive line of aseptic genderless connectors.
The new genderless AseptiQuik ® W Series connectors, with a 1 1/2” flow path, enable quick and easy sterile connections in large-volume, high-flow production and process intensification environments. The large diameter connection allows for higher flow rates, reducing the risk of flow restrictions or pressure drops that could negatively impact process performance.
The genderless design of the AseptiQuik® simplifies system integration and minimizes the risk of operator error. The connectors’ robust construction provides reliable performance without the need for clamps or fixtures. Biopharmaceutical manufacturers benefit from interchangeable 1”, 1 ¼” and 1 ½” flow solutions for full-scale bioprocessing production environments.
The AseptiQuik® W Series body is made of polycarbonate and is available in a high temperature (HT) version, suitable for autoclave or gamma irradiation applications.
Part numbers:
- AQW17016HT – AseptiQuik® W Sterile Connector, High Temperature, Fits 1 inch ID Tubing
(25.4 mm) - AQW17020HT – AseptiQuik® W Sterile Connector, High Temperature, Fits 1 1/4 inch ID Tubing
(1.25 inch, 31.75 mm) - AQW17024HT – AseptiQuik® W Sterile Connector, High Temperature, Fits 1 1/2 inch ID Tubing
(1.5 inch, 38.1 mm) - AQW33024HT – AseptiQuik® W Sterile Connector, High Temperature, 1 1/2 inch Sanitary Flange
Learn more about Qosina’s new line of AseptiQuik® W Series connectors.
Qosina is a one-stop source for single-use bioprocess components, with low minimum orders, a liberal sampling policy and bill of material kitting, all supported by regulatory documentation and backed by Qosina’s assurance of supply.
Explore Qosina’s full line of aseptic genderless connectors at www.qosina.com/aseptic-connectors.