October 8, 2020
R3 Vascular today announced closing of its Series A financing round, including new equity investments of $15 million and the conversion of $2.8 million of convertible notes. The round was led by an unnamed corporate investor and 415 CAPITAL, with participation from HBM-MedFocus LLC and Wexford Capital.
R3 Vascular reports the proceeds will support the company’s clinical trial work in developing a next generation of below-the-knee (BTK) interventional therapies for PAD.
R3 Vascular Executive Chairman Jack Springer said, “We are excited about this investment in the company’s future. The number of patients suffering from BTK vascular disease is growing significantly for many reasons, including a large global increase in diabetes and obesity. These factors, along with others, are leading to a rapid rise in critical limb ischemia (CLI), a severe form of PAD. The physician community has been waiting far too long for a more safe and effective way to treat BTK disease.”
Springer added, “I am further pleased to announce that Kamal Ramzipoor will join R3 Vascular as CEO,” said Mr. Springer. “Kamal has extensive experience in building and leading both early-stage and large medical device companies. In particular, he is a hands-on leader with extensive experience in developing and commercializing polymer-based drug/device combination products.”
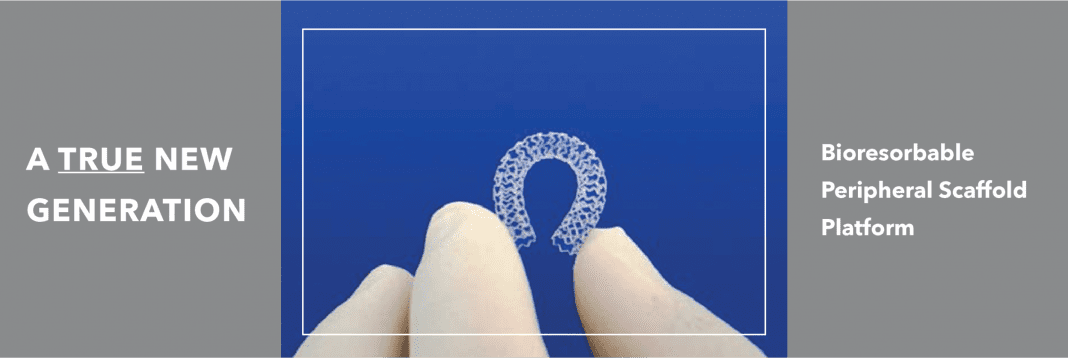
Mr. Ramzipoor added, “The unique mechanical properties and polymer technology of R3 Vascular’s proprietary bioresorbable scaffold, already validated in multiple clinical trials, offer differentiating features compared to other bioabsorbable scaffolds. It is becoming clear that the use of scaffold support offers a potential competitive advantage compared to other balloon-based therapeutic approaches in BTK interventions. R3 Vascular’s polymeric technology is the only platform that has achieved ‘DES-like’ mechanical performance at the sub-100-micron level across all scaffold diameters.”
Dr. Juan Granada, a key strategic advisor to R3 Vascular, stated that “A resorbable sirolimus-eluting device provides the best of both worlds for this type of patient: acute mechanical support, long-term lumen patency and progressive resorption allowing vessel re-intervention in case it is needed. The superior mechanical performance of the R3 Vascular bioresorbable platform has the potential to shift the therapeutic approach for patients suffering from CLI.”