Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, and Radboud university medical center (Radboudumc), a leading Dutch academic research and teaching hospital (Nijmegen, the Netherlands), have announced the positive results of a clinical study conducted by Radboudumc aimed at setting a new standard of safety and accuracy in the diagnosis of small peripheral lung lesions. The study outcomes represent a promising milestone in the global innovation effort to set a new clinical standard for detecting lung cancer at an early stage, potentially leading to improved prognoses for patients.
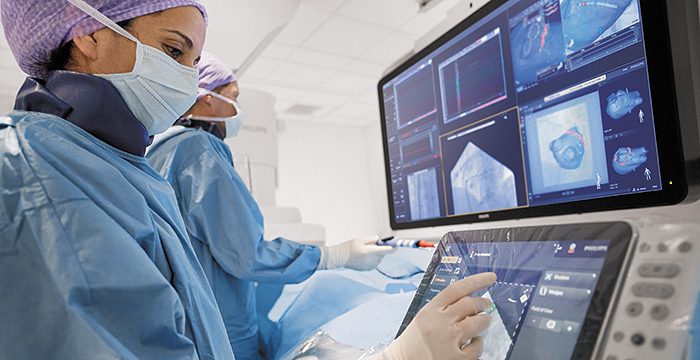
Radboudumc’s observational study reported the diagnostic accuracy and procedural radiation dose for patients undergoing an endobronchial lung biopsy supported by Philips Lung Suite – a solution that uses 3D imaging with augmented fluoroscopy to support high precision diagnosis and minimally-invasive therapy in one room. Diagnostic accuracy of 90% was reported while reducing the average total effective radiation dose per procedure by more than half from 47.5 Gy·cm2 (effective dose: 14.3 mSv) to 25.4 Gy·cm2 (effective dose: 5.8 mSv). The median long-axis diameter of the 248 lesions navigated to during the study was 13 mm (range 5-65mm). The results of the study were published in the October 2021 issue of the Journal of Bronchology & Interventional Pulmonology: Volume 28 – Issue 4 [1].
Lung cancer – the leading cause of cancer death
Early detection and diagnosis key to survivability
Challenging for clinicians to accurately navigate
A new method is image-guided endobronchial lung biopsy, during which a clinician uses live image guidance to maneuver through the airways in the lungs to advance a catheter towards the lesion. However, the airways in the lungs are a complex network that requires accurate navigation to remove the required tissue sample. Until now, image-guided endobronchial lung biopsy has only really been effective when an abnormality or tumor is located in or near one of the larger airways in the lungs, and even then it is challenging for clinicians to accurately navigate when looking at a 2D greyscale fluoroscopy image while the lungs are moving as the patient breathes.
Philips Lung Suite enables all-in-one lung cancer diagnosis and treatment. It provides advanced real-time 3D imaging with augmented fluoroscopy on the company’s Image Guided Therapy System – Azurion, combined with dedicated software. With Philips’ Cone Beam CT imaging, the X-ray detector rotates around the patient to generate a CT-like image in around five seconds, providing clinicians with a high-resolution 3D view of the target lesion and other anatomical structures. This allows the clinician performing the biopsy procedure to be continually guided by high-quality real-time imaging to advance a catheter towards the lesion through a bronchoscope. Once done, its position can be confirmed in real-time using the same imaging modality and a biopsy sample removed.
Philips has a comprehensive portfolio of lung cancer diagnosis and treatment solutions. In addition to Philips Lung Suite, the company’s Lung Cancer Orchestrator provides an integrated lung cancer patient management system for both CT lung screening programs and incidental pulmonary findings programs that manages and monitors patients every step of the way in their lung cancer screening and treatment decision journey.
References
[1] Journal of Bronchology & Interventional Pulmonology: October 2021 – Volume 28 – Issue 4 – p 262-271 doi: 10.1097/LBR.0000000000000783
[2] CA CANCER J CLIN 2021;71:7–33, Cancer Statistics 2021: https://acsjournals.onlinelibrary.wiley.com/doi/epdf/10.3322/caac.21654