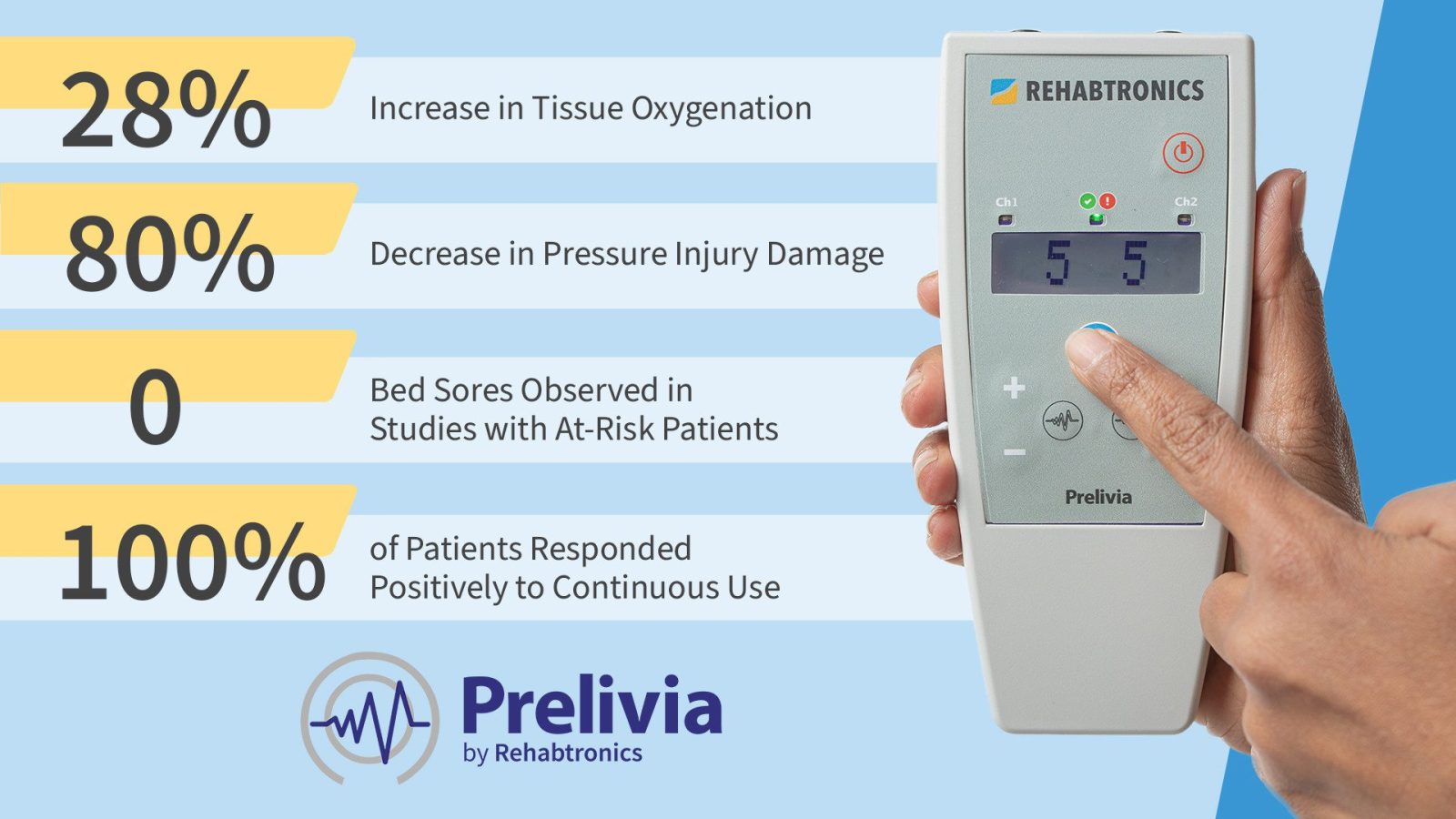
Rehabtronics has announced that Genome BC is providing $1.25 million to support commercialization of Prelivia, the world’s first neuroscience-based technology designed to protect patients against deadly pressure injuries (also known as bed sores and pressure ulcers). Despite an estimated $26 billion spent on current treatments, pressure injuries kill 60,000 people every year in the United States alone.
Rehabtronics notes the funds will facilitate free 90-day quality improvement trials of Prelivia at qualified healthcare facilities in the United States. Rehabtronics is inviting applications for the free trials now. Genome BC is allocating the funding from its Industry Innovation Program (I2), which supports promising technologies at early stages of commercial development.
“Genome BC has an impressive track record of supporting the most innovative companies in Western Canada and we are honoured by their vote of confidence in our team and technology,” said Dr. Rahul Samant, CEO of Rehabtronics. “This investment accelerates our plan to help health care facilities fight deadly pressure injuries. We look forward to implementing more quality improvement trials of Prelivia at qualified healthcare institutions in the United States.”
Prelivia has already gained U.S. Food and Drug Administration 510 (k) Clearance to promote healthy blood circulation and maintains healthy tissue in immobile patients. The neurostimulation device is the first product to address the underlying physiological pathway of pressure injury development, which is the lack of blood flow and oxygen to the tissue.
Prelivia is patented and has been scientifically proven through animal and human studies. The technology is currently part of the Protect2 multicentered randomized controlled study to further validate its effectiveness in decreasing progression and facilitating healing of pressure injuries. The Cleveland Clinic Foundation is currently recruiting participants.
“Genome BC invests in cutting-edge, innovative life sciences companies,” said Dr. Tony Brooks, Chief Financial Officer and Vice President, Entrepreneurship & Commercialization at Genome BC. “Rehabtronics has demonstrated there is an unmet need for this type of innovative product. Our investment will support the commercialization of Rehabtronics’s device to improve health care outcomes across multiple care settings, such as hospitals, long-term care facilities and in-home personal care.”
On March 17 and 18, Rehabtronics will be showcasing Prelivia at the NPIAP 2022 Conference hosted by the National Pressure Injury Advisory Panel in Wesley Chapel, Florida.