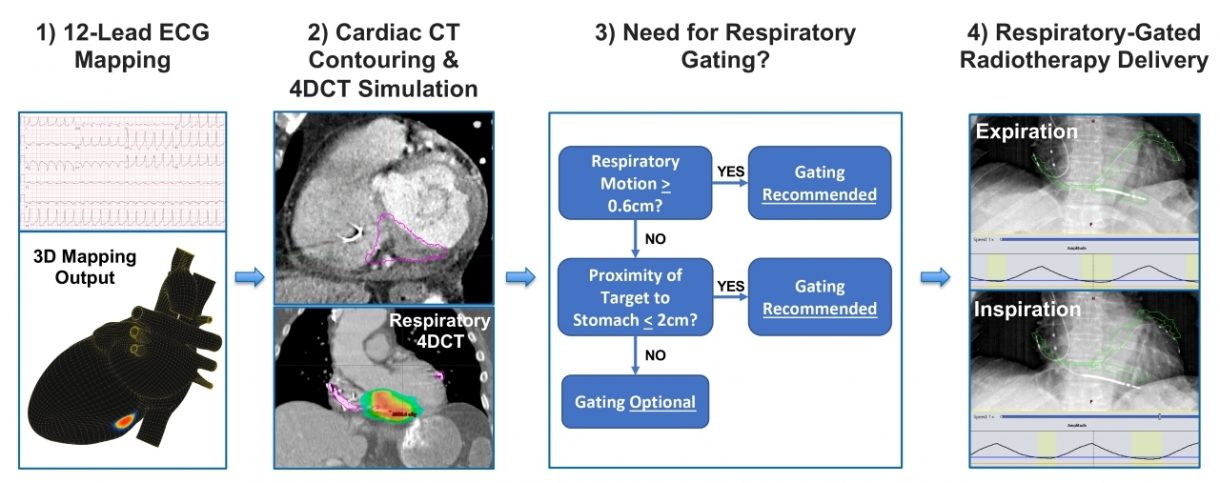
Vektor Medical, Inc., a private company founded to develop the next-generation in arrhythmia mapping systems, today announced the publication of results of a dual-site study involving its non-invasive vMap™ system during stereotactic ablative radiotherapy (SAbR), in the peer-reviewed journal, Heart Rhythm O2.
The prospective study, led by Dr. Gordon Ho, an Assistant Professor of Medicine and Board-Certified cardiac electrophysiologist from the University of California San Diego, utilized a new non-invasive workflow involving vMap™. The paper described vMap’s role in facilitating arrhythmia “target planning via 3D visualization of [ventricular tachycardia (VT)] exit sites with precision appropriate for non-invasive VT ablation.” Additionally, unlike other workflows which require the use of a mapping vest or concurrent CT scan, the vMap™ workflow requires “only the digital 12-lead ECG data of the ventricular arrhythmia.”
“SAbR is an emerging therapy that is showing great potential for treating refractory VT, however the current workflow is complicated and there are safety concerns for patients with significant cardio-respiratory motion and target sites near the stomach,” stated Dr. Ho. “We were able to show that by incorporating the non-invasive vMap™ into radio-ablation planning that we could safely ablate VT, including in those more challenging cases. Further studies will be needed, but this is an exciting first step to improve safety and efficacy of SAbR therapy.”
In the study, non-invasive mapping using vMap™ was successfully performed in all patients, identifying 4.2 ± 2.3 VT morphologies per patient, with a mean mapping time of 1.1 ± 0.1 minute per VT morphology. A total of 7 distinct VT morphologies had previously been mapped during an invasive electrophysiology study, and vMap™ was able to localize the VT exit within the exact cardiac segment in all 7 instances (100%). The study concluded that the non-invasive workflow, using vMap™’s 12-lead ECG mapping, “may help facilitate the radioablation planning workflow and provide short-term safety and maintaining efficacy during SAbR therapy in patients with advanced structural heart disease and refractory VT.”
vMap™, currently under regulatory review, aims to be the first non-invasive mapping tool that uses only 12-lead ECG data to help physicians rapidly locate cardiac arrhythmia activation sites with precision. Using advanced, proprietary algorithms, the vMap™ System turns ECG data into 2D and 3D cardiac maps of both stable and unstable rhythms in all four chambers of the heart, septum, and outflow tracts. Designed to improve first-pass ablation success and reduce procedure time, the streamlined system empowers physicians to efficiently identify target sites while reducing risks associated with invasive mapping techniques.
“While vMap™ was designed to assist the physician with traditional catheter-based arrhythmia therapy, it is exciting to see the value vMap™ may add to this entirely non-invasive SAbR planning and treatment. It is our goal to redefine arrhythmia mapping for all ablation therapies by providing rapid, non-invasive mapping for efficient and accurate identification of hotspots in all four chambers,” said Mike Monko, CEO of Vektor Medical.
Dr. Ho was recently awarded The Schulman Research Award for his outstanding work in cardiovascular medicine. This study is part of his research into how the use of novel cardiac imaging techniques can improve the accuracy of non-invasive ablation therapy of ventricular arrhythmias.