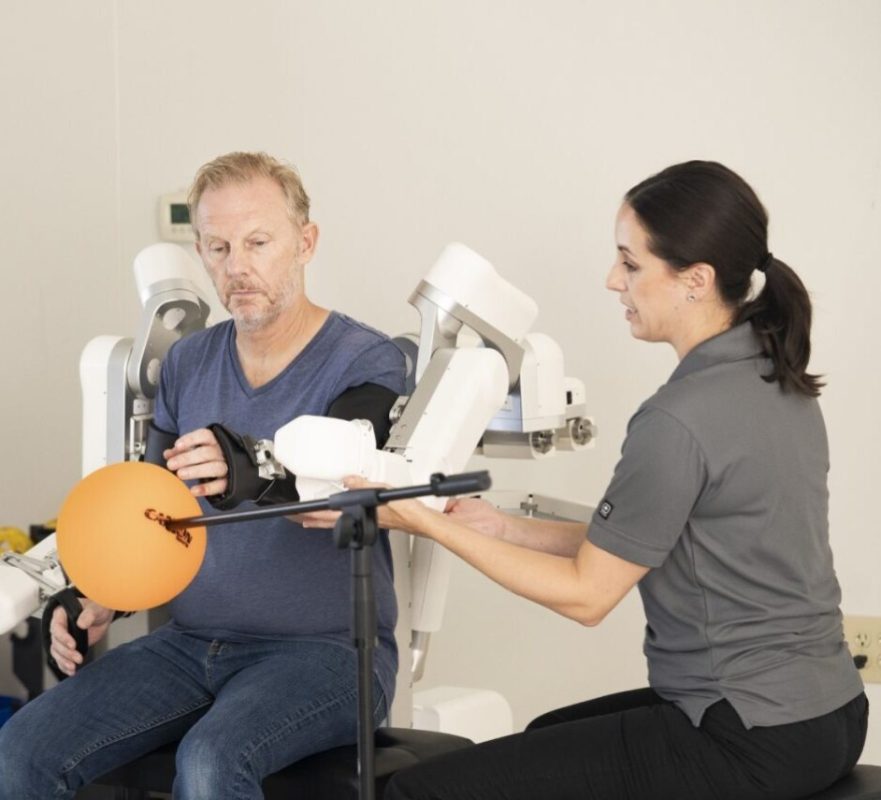
Harmonic Bionics announced today that the first Harmony SHR® exoskeleton in Hong Kong has been delivered and installed at the Chinese University of Hong Kong (CUHK) Medical Centre to be utilized in the hospital’s rehabilitation program. This marks the very first international placement for Harmonic Bionics and the first in the Hong Kong region with the company’s distributor, Healthlink Holdings.
Harmony SHR is a bilateral upper extremity exoskeleton with a biomechanically matched shoulder designed to allow a larger and more natural arm range of motion for patients recovering from neurological or musculoskeletal impairments. Harmonic Bionics announced the commercial launch of Harmony SHR last April followed by the first US installation at a top neurological hospital in Phoenix.
A high-profile dancer that suffered a spinal cord injury in an accident on stage two years ago was one of the first to use the Harmony SHR exoskeleton at CUHK Medical Centre, under the care of Professor Herman Lau, Director of Allied Health Services, and former Hospital Chief Executive of Shatin Hospital.
“The Harmony SHR exoskeleton is a highly unique robotic device that has been specifically designed for bilateral upper body rehabilitation purposes. The CUHK Medical Centre is proud to announce that it has recently acquired the first Harmony device in Hong Kong,” said Professor Herman Lau. “Patients who have been using the Harmony system have shown remarkable motivation and enthusiasm, which has resulted in some promising outcomes in a relatively short period of time.”
Harmonic Bionics CEO Mark Hunter
“This is a major milestone for our company as we work to expand our footprint and bring Harmony SHR to more providers and patients. There is tremendous opportunity for growth in Asia and this installation at CUHK Medical Centre is a great starting point to our intended success in the market.”