Most regulatory and quality professionals can think of a time when they struggled to find a specific requirement in the EU Medical Device Regulation (MDR).
Finding all the instances where a particular compliance theme is mentioned can be painstaking and time-consuming. To allow easier navigation of the MDR, RQM+ has created a tool that allows users to search the legislation for specific subject areas.
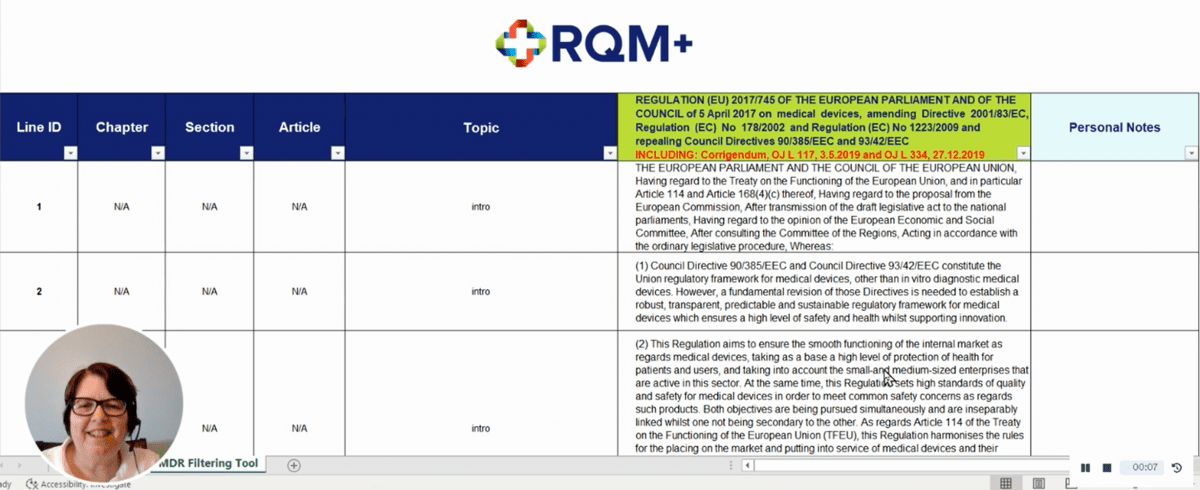
The company’s internal specialists, as well as regulatory professionals within client companies, now save considerable administrative time every day by using the tool to consult the regulation. The tool enables quick searches by topic, chapter, article, and search term. Rather than highlighting or sticky noting a paper copy, users can save notes on company interpretations of MDR sections and even individual sentences in the tool itself.
The regulatory and quality consulting firm has already shared an IVDR filtering tool, to support manufacturers working towards the EU’s upcoming In Vitro Diagnostic Regulation (IVDR). Both the IVDR and MDR tools are now freely available for medical device professionals to download and consult for their daily work.
How does it work? A demo of the RQM+ MDR Filtering Tool is available to watch here.
Dr. Jaishankar Kutty, Vice President of Clinical Services at RQM+, said, “I was recently introduced to this tool after joining RQM+ this February, and my first reaction was: it would’ve been wonderful to have this tool when we waded into the initial MDR reviews at BSI! More than any other stakeholder, Notified Bodies need to know the regulation inside-out, and this tool makes it so easy to double check requirements. It’s now sitting on my desktop for easy access and it has been an extremely useful and welcome support so far.”
Nancy Morrison, Executive Director, Regulatory & Quality Consulting Services, initially developed these tools to support the RQM+ team. “We refer to regulation on a daily basis so we’re well aware of the frustrations of trying to find specific details or to gather all the sections referring to an area of compliance. We help our clients to put in place clear and organized processes to facilitate both short- and long-term compliance, and I hope that these tools will also make the work of regulatory professionals a little more straightforward.”
The RQM+ MDR Filtering Tool is available to download here.
The RQM+ IVDR Filtering Tool is available to download here.
