Shanghai Operation Robot Co., Ltd announced the results of its initial clinical trial of ERCP (Endoscopic retrograde cholangiopancreatography) robot in biliary stent placement surgery. It is the first Robot-assisted human clinical trial of biliary stent placement surgery in the world. The surgery was conducted by Aopeng Medical.
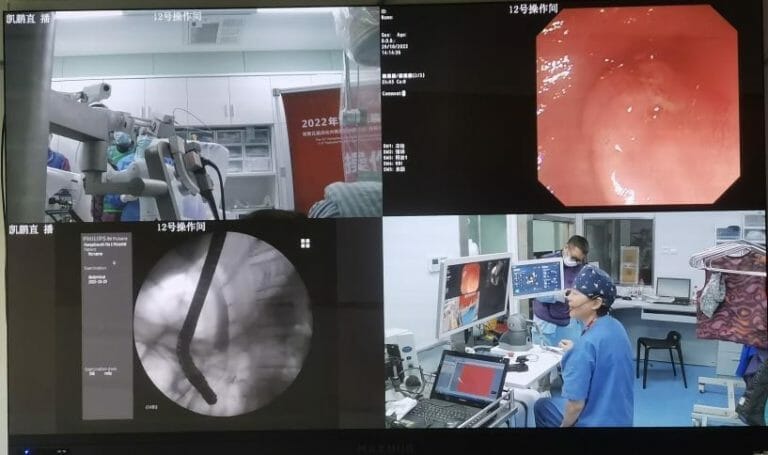
The operation was performed by the team of Professor and Dr. Xiaofeng Zhang, vice president of Hangzhou First People’s Hospital and director of the Center for Digestive Endoscopy. Dr. Zhang operated the robot outside the operating room (without wearing heavy lead), advanced the guidewire and balloon catheter in a precisely controlled manner and successfully completed the entire surgical procedure including the nipple cutter action, balloon stone removal, and accurate placement of bile duct stents.
Dr. Zhang highly values the precision performance of the ERCP robot from Shanghai Operation Robot, and the benefit for physicians in reducing radiation exposure during the surgery. This surgery was live-streamed at the meeting of the 15th Hangzhou International Symposium on Diagnosis and Treatment of Biliary and Pancreatic Diseases. Many experts in the audience watched the whole procedure and considered it a great success for robot-assisted ERCP surgery.
This trial verified the application of surgical robots in the field of natural orifice ERCP surgery and opened a new era of robotic surgery.
ERCP surgery is carried out under X-rays, so the physicians need to wear a 30-pound protective lead apron. They are suffering health risks from long-term radiation exposure and heavy lead weight. In addition, the variable orientation and opening position of the duodenal papilla requires a high level of guide wire operation and the doctors’ cooperation with the assistant. Therefore, the training of a qualified ERCP doctor and an excellent ERCP team requires the accumulation of operating time and experience. These can be easily resolved by surgical robots. In robot-assisted ERCP surgery, the physician can perform the operation remotely behind the wall, away from X-ray exposure. Furthermore, the natural stability, accuracy, and flexibility of robots can effectively reduce the learning curve of ERCP surgery and bring a better outcome to patients.
The natural orifice ERCP flexible surgical robot from Shanghai Operation Robot has a modular design, which imitates the physician working with an assistant. Its small footprint enables easy and flexible movement in the operating room. The robot adopts the principle of master-slave operation, the operation of the master stick is exactly the same as that of traditional manual operation, which greatly shortens the learning time. By operating the left and right master stick together, the physician can manipulate the knob of the endoscope handle, the forceps lifter, the water and gas button, the water absorption button, the guide wire and catheter delivery, withdrawal, and instrument exchange. The robot also includes a closed-loop mechanism for the delivery of the guide wire and instrument, which avoids the risk of slippage or blockage during the movement of the guide wire and instrument. The resistance is reproduced in real-time during the master operation, so the physician possesses the actual feeling.