Shoulder Innovations, a leader in shoulder replacement technology, today announced the United States Patent and Trademark Office (USPTO) has granted nine new patents over the past 18 months that significantly expand the Company’s intellectual property portfolio.
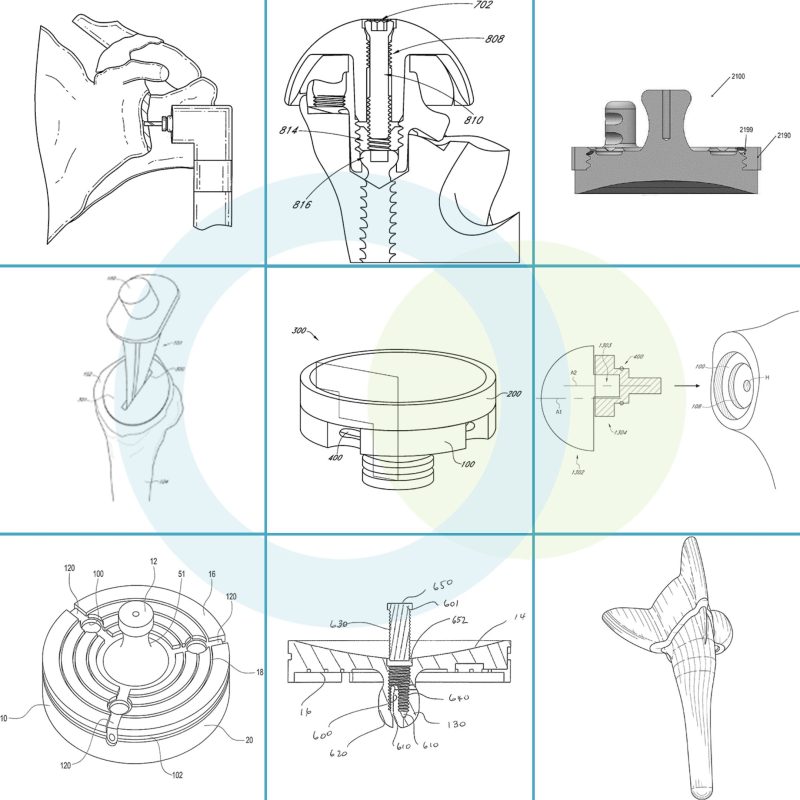
The newly granted patents cover key areas within shoulder replacement and less invasive surgical techniques, including, among others, glenoid preparation in a single step, total reverse shoulder systems and methods, and porous coated convertible glenoid.
“These recent grants further strengthen key patent families that are foundational to our technology, and we are pleased the USPTO continues to recognize our meaningful innovation in the shoulder arthroplasty segment,” said Rob Ball, CEO of Shoulder Innovations. “This noteworthy expansion of our IP position represents the culmination of over 10 years of research and development, and we are proud of our team for their continued dedication to creating practical solutions for shoulder surgeons and advancing patient outcomes.”
Over the past 20 years, Shoulder Innovations has developed a robust intellectual property portfolio around the Inset Glenoid technology and brand that is marked by a continued long life prior to expiration. Specific patents among the newly granted awards adding to the Company’s existing strong IP foundation include:
Glenoid Preparation in a Single Step
- This patent covers a groundbreaking method of implanting a glenoid implant, which involves reaming two cavities in the glenoid in a single step.
Total Reverse Shoulder Systems and Methods
- This patent features a reverse shoulder system with a scapular glenoid baseplate and humeral stem, where the baseplate’s axis is angled relative to the stem’s axis.
Porous Coated Convertible Glenoid
- This patent covers shoulder replacement components and methods utilizing porous fixation rings, enabling the switch from anatomic to reverse articulating surfaces without changing the fixation components.
“Shoulder Innovations‘ commitment to advancing shoulder replacement technology is further evidenced by these patents,” said Dr. David Fehnel. “As a shoulder surgeon, I’m thrilled to see these technological developments rapidly making their way into the marketplace, with the goal of helping us provide even more innovative surgical care for our patients.”