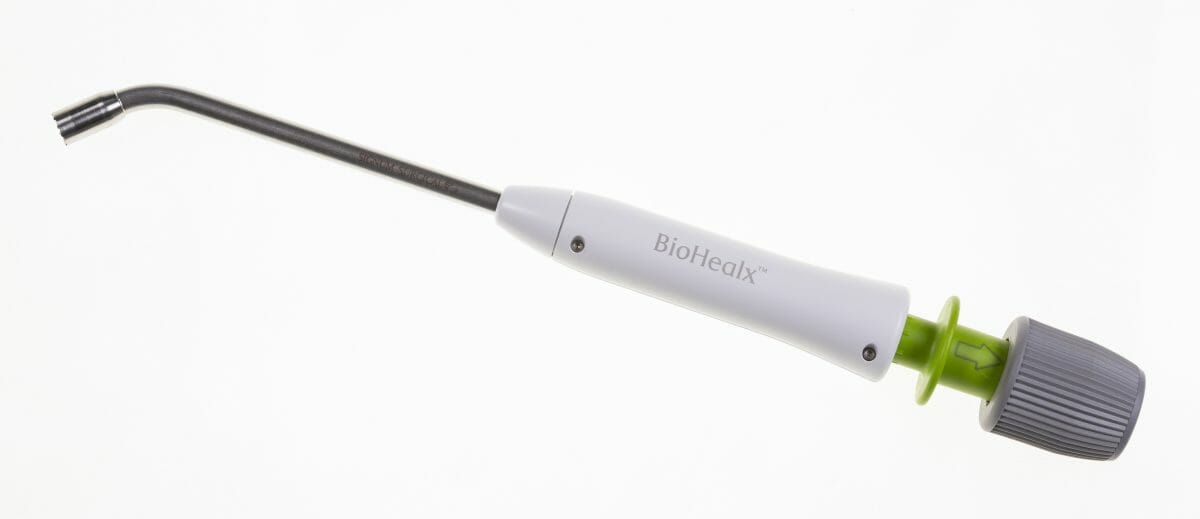
Signum Surgical, (‘Signum’ or ‘the Company’) the clinical stage medical technology company developing innovative solutions to treat colorectal diseases, announces the completion of its single-arm, non-randomized, clinical trial to evaluate the safety and efficacy of its BioHealxTM device for the treatment of anal fistula.
The multi-centre clinical trial, led by four investigators, treated a total of 32 patients in the adult male and female patient population with BioHealxTM, who had experienced recurrent anal fistula from at least one previous failed procedure. Final follow up assessments have been completed on the 32 patients over a period ranging from 13 to 40 months. This clinical trial data is currently being compiled for submission of a De Novo classification request to the U.S. Food and Drug Administration (FDA) and scientific publication in due course, which would pave the way for bringing BioHealxTM to market.
As a novel treatment option, with no legally marketed predicate device, Signum Surgical will submit a De Novo request to classify BioHealxTM,. A DeNovo request is a risk-based classification process for which general controls alone, or general and special controls, provide reasonable assurances of the safety and efficacy for its use in the treatment of anal fistula.
Peter Ónody, Colorectal Surgeon and Principal Investigator of the clinical trial, commented: “I am excited at the potential of BioHealxTM as a novel surgical option for the treatment of anal fistula and, in particular, its ease of use for physicians. This treatment has the potential to eliminate the need for multiple surgeries and substantially reduce surgical trauma and the rate of fistula recurrence, while reducing costs for patients and the overall health care system.”
Moshe Zilversmit, Co-Founder and CEO of Signum Surgical, commented: “The completion of our clinical trial marks a significant milestone for Signum Surgical and our BioHealxTM device. We look forward to submitting our De Novo classification request to the U.S. FDA and the scientific publication of the data in due course, as we seek to bring this novel treatment to market for the benefit of patients, surgeons, and the healthcare system.”
Headquartered in Galway, Ireland, Signum Surgical is a clinical stage, ISO 13485 certified, company focused on developing minimally invasive technologies to treat colorectal diseases, firstly introducing the BioHealx™ technology to address the treatment of anal fistula. This painful colorectal condition affects one in 5,000 people worldwide1 and in the United States over 90,000 surgeries are performed annually to treat anal fistula2. Current treatment options are often unsuccessful, which frequently result in inadequate or slow healing, a high risk of incontinence, and repeat procedures.
Developed in collaboration with expert colorectal surgeons, BioHealxTM will enable surgeons to treat anal fistula with a minimally invasive outpatient procedure, featuring a single use, bioabsorbable implant that is designed to close the fistula tract and dissolve in the body after treatment. The single-operation approach is designed to promote healing, prevent fistula recurrence, and pro