Sirius Medical, a leader in tumor navigation technology, announced today the closing of its second round of series A financing. The undisclosed amount will be used to accelerate expansion into new markets, including the United States, and to invest in technology innovation and product roadmap development.
The funding was provided by BOM Brabant Ventures, Holland Capital, Curie Capital, Sirius Medical team, and angel investors.
“I am grateful for the enduring trust and commitment provided by our investors and the team at Sirius Medical,” says Bram Schemers, CEO Sirius Medical. “This financing round enables us to execute and accelerate our ambitious growth plans as well as further develop our innovation pipeline to deliver superior navigation technology to physicians and patients worldwide.”
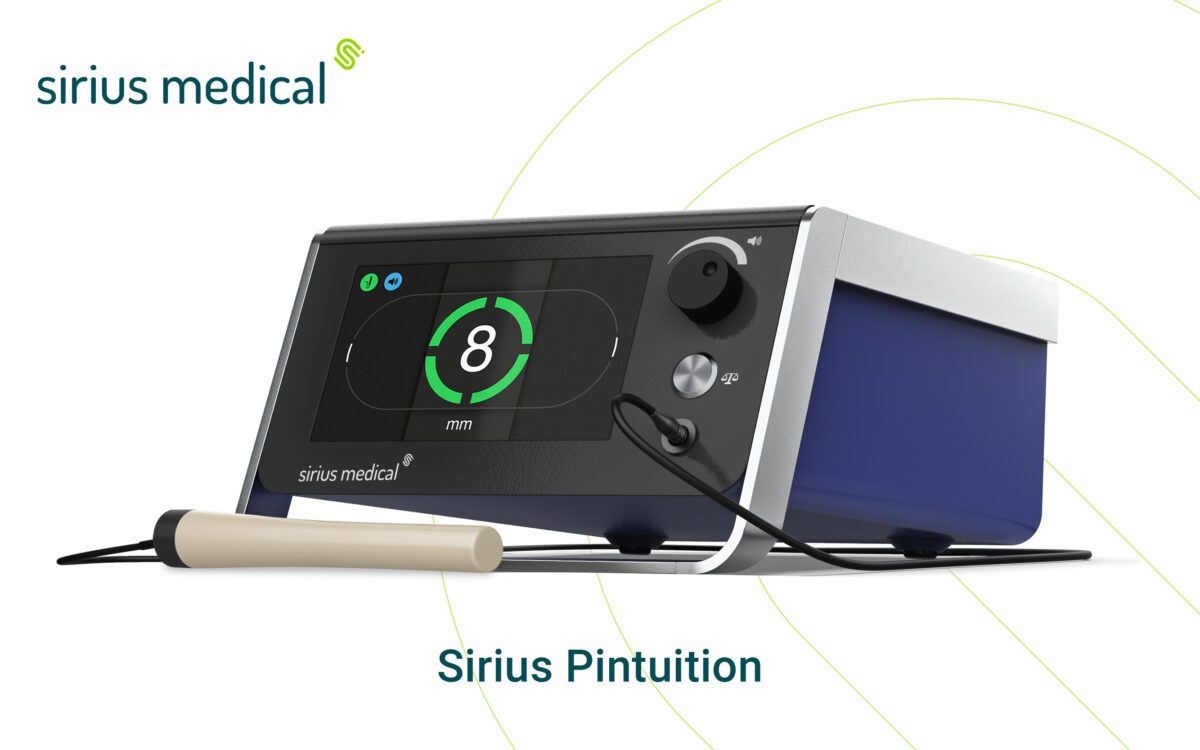
Recently, Sirius Medical launched its advanced GPSDetectTM software at the 40th European Society of Surgical Oncology conference in Lisbon. Sirius Pintuition® powered by GPSDetect provides surgeons with real-time directional guidance using audio and visual feedback to locate tumors more easily and accurately.
“It is amazing to see the speed of innovation and the adoption of Sirius Pintuition in such a short amount of time,” says Jan-Frens van Giessel, partner at Holland Capital. “As investors we are proud to support this venture that aims to bring optimal care to patients with breast cancer worldwide.”