SKIA Co., Ltd. (CEO Lee Jong-Myung) has introduced an innovative technology that will lead to an evolution in surgical preparations and take procedures one step further.
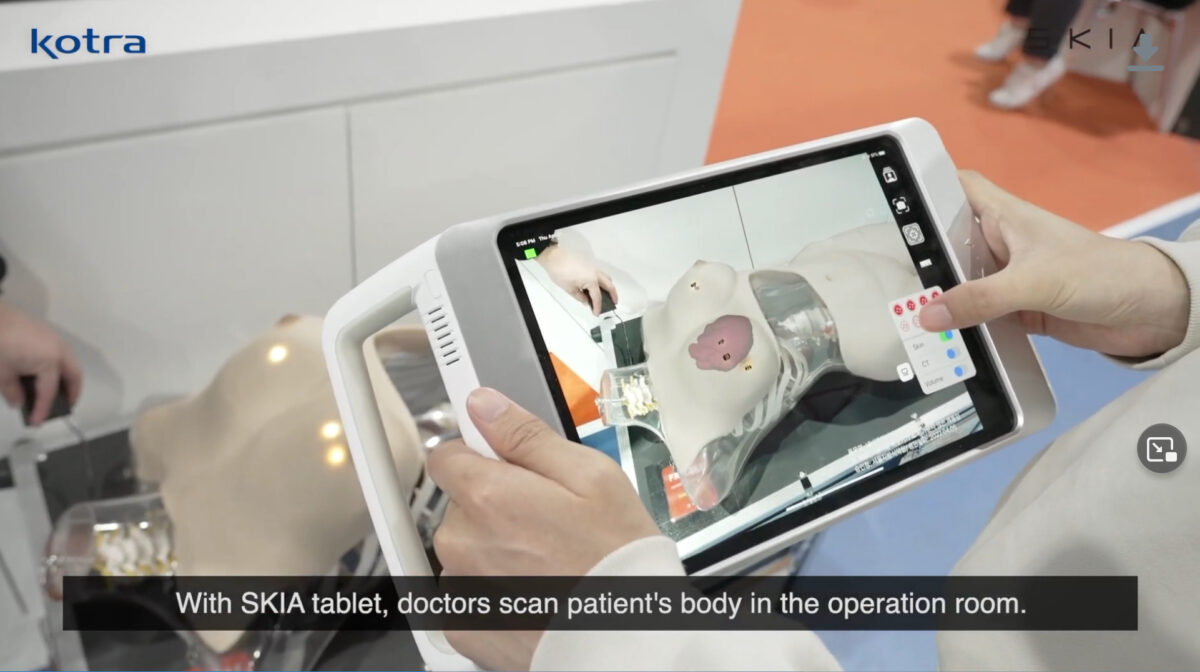
SKIA_Breast is a digital surgery guide solution based on augmented reality (AR) that superimposes a patient’s medical images (like CT or MRI) with a real-time camera view of the surgeon. The surgeon can check the location or shape of tissue by examining an image which is projected onto the patient’s skin which could not normally be seen with the naked eye. Without having to visualize a two-dimensional image on the monitor as three-dimensional, the surgeon can concentrate more on the operation. Therefore, the accuracy and stability of the procedure are greatly improved.
For any successful surgery, it is very important to accurately capture the location and size of a lesion and to be able to reach the lesion by causing as little injury to the surrounding tissues as possible. This is one reason why a surgeon’s experience and the sensitivity and control in their fingertips have long been considered more important than anything else, despite the introduction of various cutting-edge diagnostic equipment or robots.
SKIA_Breast consists only of a tablet and a case with a 3D sensor, making it portable and easy to use. In addition, there is no need for a workstation with various sensors that are large and sensitive to shock or vibration.
SKIA_Breast can see through the human body, so it helps to reduce any deviation of skill level according to surgical experience. Additionally, for patients, radiation exposure from CT scans may be reduced. It can also replace wire localization, which is for patients with small lesions that cannot be identified by touch.
The biggest strength of the SKIA_Breast is that it is “markerless”. Most current AR technologies applied in surgery need multiple markers to be physically implanted in bone tissue such as the spine or skull. Since SKIA_Breast uses no markers, additional procedures to implant markers are not required. Moreover, through its further development, it can be used for education, patient explanation, and additional surgeries.
SKIA Co., Ltd. is a medical extended reality software development startup which was established on September 1, 2018. The company released a patent for a medical image augmented reality system in Korea in October 2019. Also in September 2020, they successfully registered a patent in the USA (US 10803608 B1). In October 2021, they won the Johnson & Johnson QuickFire Challenge 2021. Currently, SKIA_Breast is in a clinical trial phase with Ewha Womans University Hospital.
Lee Jong-Myung, the CEO of SKIA, said, “We are also working with other hospitals to expand application of our solution to procedures such as facial reconstruction and lithotripsy. In the future, we will develop a solution that can be applied to other organs such as liver and pancreas.”