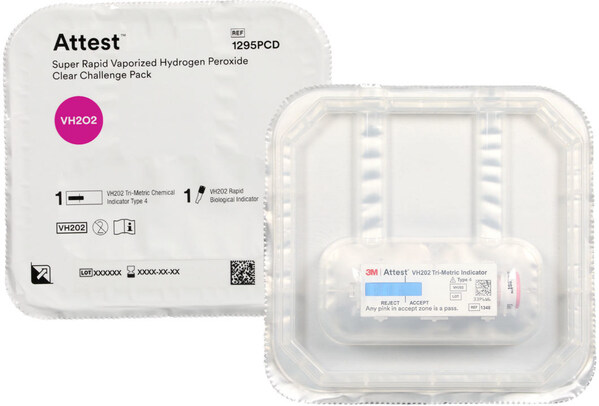
Solventum, a global MedTech leader at the forefront of infection prevention innovation, today announced the launch of its Attest™ Super Rapid Vaporized Hydrogen Peroxide VH2O2 ) Clear Challenge Pack. The ready-to-use test integrates two previously FDA-cleared indicators – a biological indicator (BI) for confirming microbial neutralization and a chemical indicator (CI) for verifying proper sterilizer function – into a single-use test pack with a transparent container.
Medical instrument sterility is a critical component in the fight against hospital-associated infections, which impact one in every 31 hospital patients in the U.S.4 The practice of every load monitoring – using dual indicators (both BIs and CIs) to verify the efficacy of every sterilization cycle for each batch of instruments – is recommended by industry guidelines and expert consensus to help minimize patient safety risks.
This unique combination makes the Attest™ Super Rapid VH2O2 Clear Challenge Pack the first and only preassembled VH2O2 test pack that is U.S. FDA-cleared for routine monitoring across multiple sterilizer brands, models and cycle types*, offering:
- Convenience: preassembled, ready-to-use pack eliminates the step of assembling testing pouches with separate indicators, saving time and mitigating the risk of errors inherent to manual preparation, like incorrect indicator placement or omission of an indicator.
- Accuracy: engineered to more accurately simulate the environment within a wrapped set of surgical instruments during a VH2O2 cycle.5 This helps to provide a realistic challenge to the sterilizer’s ability to neutralize potentially harmful microorganisms, providing greater assurance that a “pass” result reflects effective sterilant penetration and conditions within the load.
- Simplification: integrated, all-in-one design helps streamline inventory management by reducing the need to order BIs, CIs and peel pouches individually.
“Innovations that make every load monitoring easier to adopt, more reliable, and faster across sterilization modalities empower healthcare facilities to consistently uphold the highest patient safety standards while supporting the increasing productivity demand of modern sterile processing departments,” said Sharon Greene-Golden, past president of the Healthcare Sterile Processing Association (HSPA).6 “Many of today’s advanced surgical instruments simply cannot tolerate high-temperature steam so, the availability of validated low-temperature sterilization processes like VH2O2, coupled with rigorous monitoring, is part of what enables these sophisticated instruments to be used safely.”
The pack’s transparent design allows sterile processing professionals to immediately inspect both the CI and BI’s process indicator located on its cap post-cycle without breaching the pack’s seal. In addition to the instant CI-result, this enables the BI’s physical integrity to be confirmed visually before incubation. Confidence that the BI is undamaged and correctly processed at this early stage of the process can contribute to streamlined workflows and timely instrument load release decisions.
“In the high-stakes environment of sterile processing where patients’ lives are at stake, both speed and accuracy are non-negotiable,” said Doug Bartlett, senior vice president of the company’s Infection Prevention & Surgical Solutions business. “Solventum innovations equip dedicated professionals with intuitive, cutting-edge resources that help them to safeguard patient health, reflecting our drive to continually support elevated standards of care in critical settings.”
The Attest™ Super Rapid VH2O2 Clear Challenge Pack is available immediately in the U.S. through authorized Solventum distribution partners. It is slated for a global rollout throughout 2025,7 underscoring Solventum’s unwavering commitment to advancing sterile processing practices worldwide.