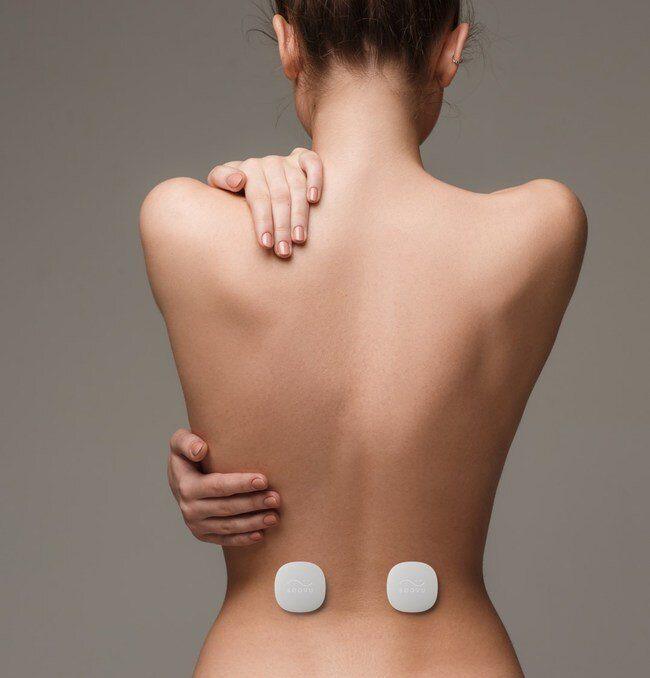
Soovu ™ Wearable Pain Relief, is a unique “therable” (wearable medical device and app) to treat chronic pain.
In the U.S. more money is spent on pain treatments and lost productivity than diabetes, cancer and heart disease combined. Clearly opioids are not the answer, and recently the FDA required NSAIDs, like Aleve and Advil, to include heart attack and stroke warnings on their labels. According to Chuck Chabal, MD and Co-Founder of Soovu Labs, “In the COVID era, pain complaints are up by 50%, as are comorbidities of anxiety, stress and depression. The need for transformation in the management of pain has never been greater.”
With funding from the National Institutes of Health, Dr. Chabal and fellow researcher and Co-Founder Dr. Peter Dunbar developed a drug-free, wearable for on-the-go relief. These doctors discovered that pulsing high levels of heat on the skin desensitized the “TRPV1” receptor, and in so doing provided immediate and long-lasting relief of chronic pain1. Published, independent clinical studies confirmed that Soovu significantly reduced chronic pain compared to a placebo device. The Soovu companion app provides digital coaching to reduce stress and anxiety associated with pain, and sends results to the cloud for machine learning for personalized treatments. The device and app uniquely address both physiological and psychological pathways to pain relief.
Soovu Labs is currently completing a round of funding to scale up production. With its launch of Soovu Wearable Pain Relief in fall 2021, the company is poised to reduce the pain of millions of Americans and increase the possibilities in their lives.
Soovu Labs, Inc. is on a mission to transform the treatment of chronic pain without drugs, invasive procedures or surgery. Their first product, Soovu Wearable Pain Relief, will target treatment of chronic back and neck pain.
1 Soovu Wearable Pain Therapy is indicated to provide topical heating for the purpose of elevating tissue temperature for the temporary relief of minor muscle and joint pain and stiffness; the temporary relief of joint pain associated with arthritis; the temporary relief of muscle spasms, minor sprains and strains, and minor muscular back pain; the temporary relief of menstrual discomfort; the relaxation of muscles; and the temporary increase of local circulation where applied.