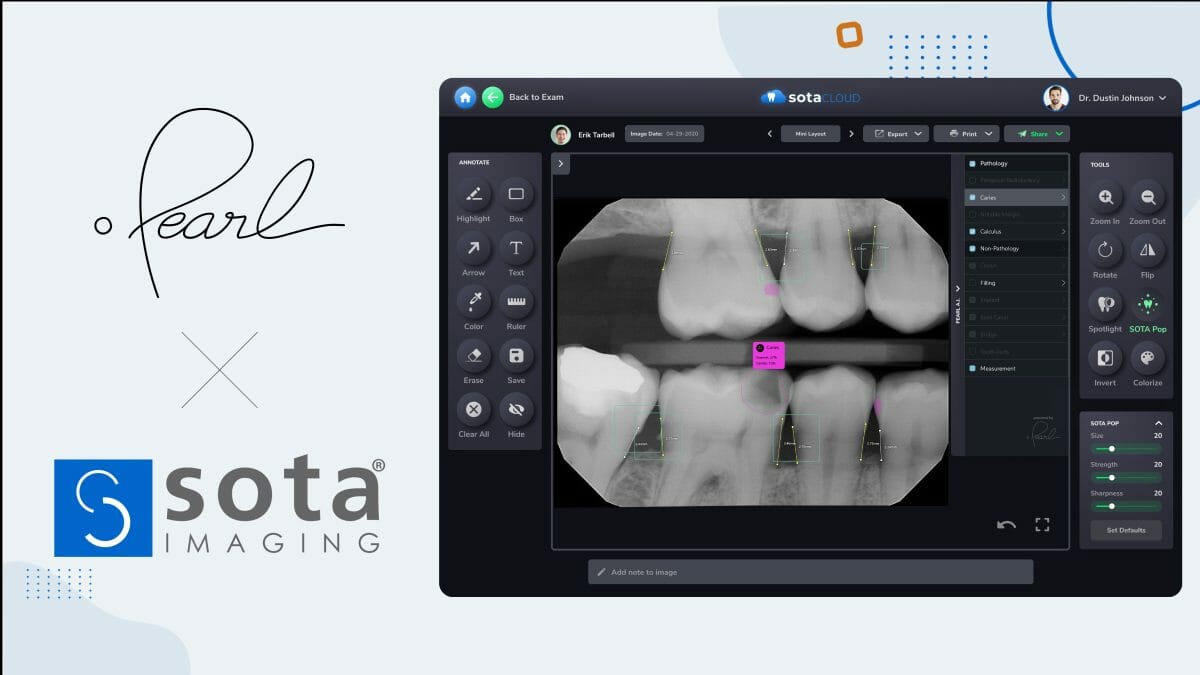
Pearl, the global leader in dental AI solutions, and SOTA Imaging, the leading manufacturer of dental imaging hardware and software, announced today a partnership to integrate Pearl’s FDA-cleared Second Opinion® real-time clinical pathology detection artificial intelligence (AI) solution with SOTA’s popular SOTA Cloud® dental imaging software.
Native support for Pearl’s chairside AI within SOTA’s cloud-based imaging interface means that, for the first time, dentists in the United States can conveniently capture, display, and perform AI-assisted clinical evaluation of dental radiographs within a single software platform.
“Together with SOTA, we are pushing the envelope of dental imaging further toward a fully AI-powered dental future,” said Ophir Tanz, Pearl’s founder and CEO. “AI will be a basic utility in dentistry and we see obvious advantages to making that utility an integral feature of the tools that dentists are already using. Through this partnership, dentists using SOTA Cloud® can seamlessly access Pearl’s AI capabilities to obtain better, more consistent insights from patient imagery and present cases to patients more effectively –– leading to better outcomes, a higher standard of care and more productive patient communication.”
Pearl’s Second Opinion® is a clinical AI system cleared for use as a patient-facing radiologic aid to dentists in over 90 countries. With native Second Opinion® integration, dental practices using SOTA Cloud® –– the robust cloud-based dental imaging software which SOTA introduced last year to complement its line of state-of-the-art intraoral cameras and X-ray image sensors –– can view automatic AI detections of most conditions commonly found in dental X-rays.
“Understanding the needs of clinicians and ensuring that dentists are able to focus solely on providing the best possible patient care has always been the top priority when developing our software,” said SOTA founder and president Brian Kim. “Our partnership with Pearl speaks directly to that focus by giving practices using SOTA Cloud® push-button access to the most innovative AI technology available for streamlined patient communication and improved image-based diagnostic evaluations.”
To register for SOTA Cloud® with fully-integrated AI capabilities, visit: https://info.sotaimaging.com/sotaxpearlpartnership
To learn more about Pearl’s Second Opinion®, visit: www.hellopearl.com/products/second-opinion.
To learn more about SOTA Cloud®, visit: www.sotaimaging.com/sota-cloud