Intense chronic low back pain (CLBP) is a global epidemic that plagues over 266 million people worldwide. We are not referring to the kind of sore back that you have after a long flight or weekend sleeping on your friend’s sofa, but rather the grinding, “drops you to your knees” pain that prevents you from going to work or school. It’s the kind of pain that keeps moms from playing with their kids and dads from coaching Little League. It’s the kind of pain that employers dread due to the costs associated with high absenteeism and lost productivity. This cruel and unrelenting condition is also the leading cause of opioid addiction in the United States and accounts for the single largest expenditure in all of healthcare. When pain like this invades one’s life, it steals everyday joy and replaces it with a tsunami of lost opportunities.
Impact of Current Treatments on Outcomes and Cost
It’s no wonder that sufferers of chronic low back pain seek help wherever they can find it. Treatments range from pain medications and physical therapy to chiropractic care and injections. In 635,000 cases per year in the US, the road eventually leads to spinal fusion surgery. At an average range of $37,000 to $75,000 per procedure, spinal fusion surgery is an expensive and highly invasive approach to finding relief. What’s worse is that the success rate of these fusion surgeries hovers between 47% and 54% as measured by the patient’s indication that they have achieved significant pain relief post-surgery.
Spinal anatomy is highly complex, and multiple factors can contribute to the cause of pain, making it challenging for the treating physician to pinpoint a single source. A physician may perform perfect surgery on two levels, but omit treatment for the adjacent level that is a major source of pain. This opaque picture of the source of pain leads to treatments that simply miss the mark, which leads to poor outcomes and $2.2 billion spent on spinal revision surgeries in the U.S. each year.
This begs the question: how did the doctor miss a painful disc during examination? The issue is that not all pain generators are visible on an MRI or other imaging modality, like a CT scan or an X-ray.
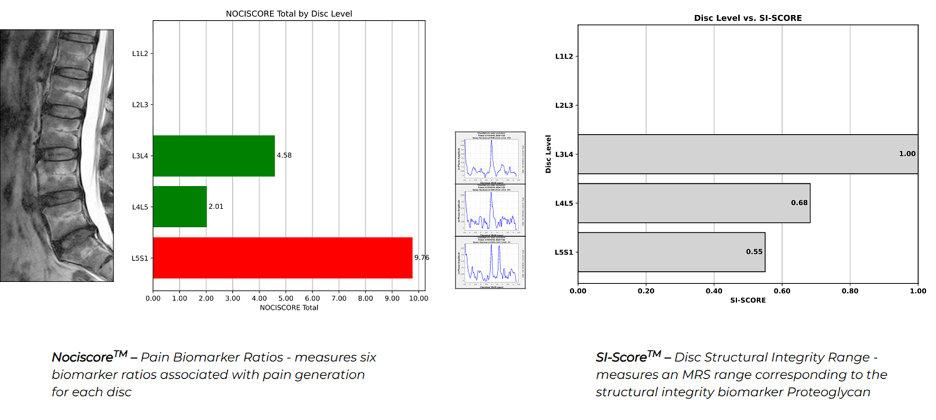
Seeing the Invisible: The Science Behind Nociscan
The good news is, we can objectively measure the biomarkers inside the lumbar discs that, in a certain ratio, correlate to pain as compared to provocative discography, the invasive gold standard for determining discogenic pain. The disconnect between cost and positive outcomes led researchers at the University of California, San Francisco (UCSF), to discover that using MR Spectroscopy, when a disc contains high levels of acids and low levels of structural integrity biomarkers, the disc is highly likely to be a source of pain. MR Spectroscopy does not create a picture out of the raw data derived from the MR machine; rather, it quantifies the biomarkers contained within the area of interest assigned to it by the MRI Technologist. This finding led to the development of Aclarion’s technology, Nociscan.
Nociscan leverages augmented intelligence to translate highly complex data from the MR Spectroscopy acquisition into an easy-to-interpret PDF report. Each disc is evaluated in the cloud through our SaaS based model, where the content of Alanine, Lactic Acid, and Propionic Acid is measured against the level of Proteoglycan, a biomarker that indicates the strength of the disc. In combination, the evaluation of pain-generating biomarkers and structural integrity biomarkers helps physicians choose the right levels to operate on. The objective information provided by Nociscan can help the physician pinpoint the pain or “See the Invisible.”
Commitment to Clinical Evidence
Evidence shows that when a procedure matches the Nociscan results, outcomes jump to 97% positive compared to 54% when Nociscan is not utilized. This is a fantastic improvement not only for each patient who gets the procedure planned with Nociscan input, but also for the entire health system. An independent healthcare economist, Dr. Leslie Wilson, published a peer-reviewed paper in ClinicoEconomics and Outcomes Research with the following conclusion: This study demonstrates cost-effectiveness dominance of MR Spectroscopy with the Nociscan diagnostic algorithm over PD for identifying CLBP surgical candidates. MR Spectroscopy provides significant cost savings and leads to better surgical outcomes, making it a preferred choice for insurers and health systems.
With respect to evidence, Aclarion is not stopping there. We have launched the CLARITY trial, a multi-site, multi-surgeon national randomized controlled trial designed to prove that using Nociscan improves patient outcomes. The trial will enroll 300 patients who are heading to surgery and are randomized 1:1 between the surgeon being blinded to the Nociscan results and unblinded. We will measure outcomes at 3,6,12, and 24 months, with 12 months being the primary outcome. We will evaluate if, when Nociscan is followed, the outcomes improve vs when Nociscan is not followed.
Several other real-world evidence studies are also underway around the world, so in time, the preponderance of peer-reviewed publications will create the foundation for reimbursement coverage decisions, leading to full market launch in geographies where coverage exists.
Real-World Impact: London Experience
In the London market, three of the top four private insurance companies have already approved payment for the use of Nociscan at the London Clinic. This has contributed to nice early growth in scan volume and revenue from that market. To date, we have completed more than 1,200 commercial scans and are available at multiple centers throughout the United States and the United Kingdom. Our expansion plans are quite simple: lead with evidence and work to show the payer community that the adoption of Nociscan in the treatment paradigm of CLBP patients both improves outcomes and reduces costs. From there, we will be expanding our commercial team to work with health systems and treating physicians to bring this AI-enabled SaaS model of evaluating individual biomarkers to improve patient outcomes to the full standard of care.
Kathryn, a patient who received a successful intervention for her pain, said, “It gave me my gym back, it gave me my job back, it gave me my friends back, it gave me my LIFE back.”
Our purpose is to help millions of people, just like Kathryn, experience positive results when they seek out physicians to find and alleviate the pain that nobody can see.
Editor’s Note: Brent Ness is the CEO, President, and Director of Aclarion. With over 25 years of medical device and healthcare technology experience, he’s held various executive and senior leadership positions, including recently as CCO of Cleerly, Inc., where he built key industry partnerships with Canon Inc. to drive adoption of their AI-enabled cardiology solution.
Previously, Mr. Ness was the COO of Mighty Oak Medical, whose principal products progressed from pre-FDA clearance through an international full market launch of their platform called FIREFLY. Mr. Ness also served as CCO of Heartflow, Inc., where he led the company from pre-FDA clearance through a global expansion of early adopter sites. He also previously served as President of ProNerve, LLC, where he led a roll-up of the highly fragmented Interoperative Nerve Monitoring Industry.
Additionally, Mr. Ness has held senior leadership roles at Medtronic, GE Healthcare, and Philips North America. He is a graduate of the University of North Dakota with a BS in Business Administration and received his MBA from the University of Colorado.
