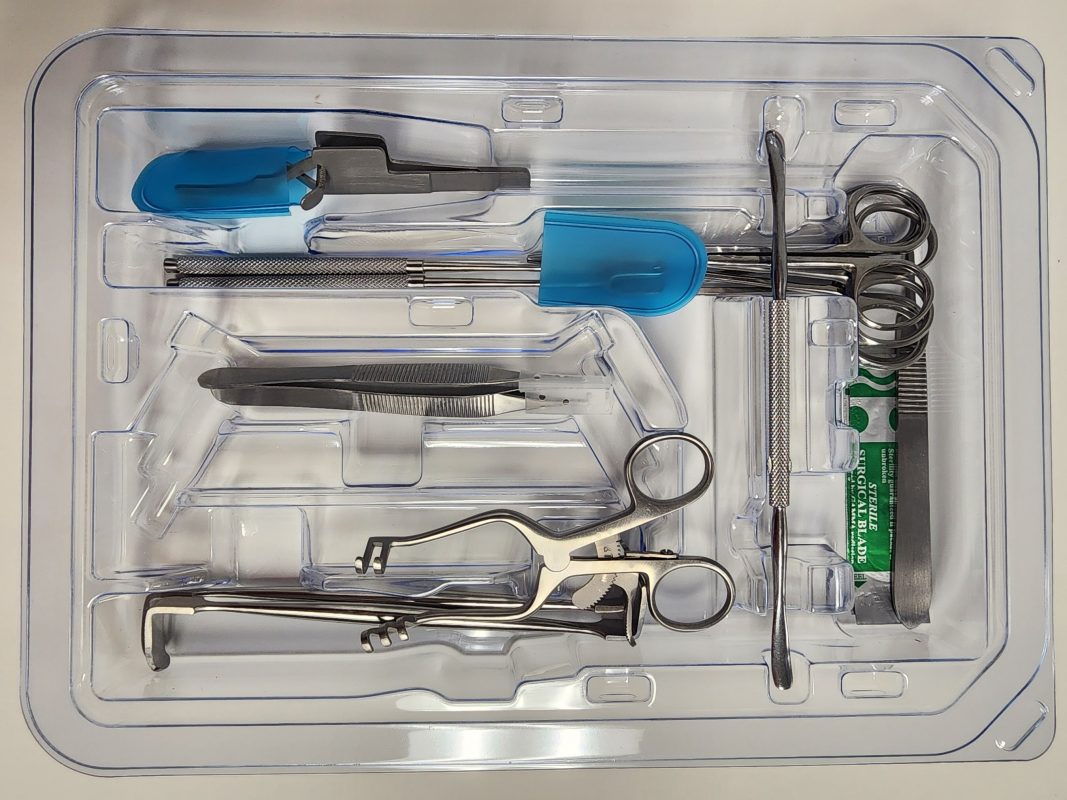
Spartan Medical, Inc., a veteran-owned medical solutions company, has expanded the industry’s most extensive range of single-use, sterile, pre-packaged (SSP) surgical instruments for use in the Veterans Administration (VA), the Department of Defense (DoD), and select civilian hospitals and Ambulatory Surgery Centers (ASCs) nationwide. Spartan Medical products portfolio of single-use, sterile med tech includes micro and minor surgical convenience kits, kerrison rongeurs, spinal and general surgical retractors, dural repair kits, synthetic biologics, and a wide range of orthopedic pre-sterilized implants and devices.
In addition to their well-documented ability to reduce Surgical Site Infections (SSI), this set of innovative SSP solutions can substantially reduce the number of reusable trays and instruments per surgery, driving increased Operating Room (OR) throughput, lowering overall costs to re-sterilize while reducing environmental impact.
As Spartan Medical’s President Vince Proffitt explained, “when you have the opportunity to not only make the surgery safer for the patient but also improve the efficiency of a hospital’s whole surgical system, converting to single-use sterile instruments is a clear winner. Driving surgical innovation doesn’t always take the form of a flashy new implant — the most complex part of any surgery is the massive support system required to put the right tools in the right hands, well before they are needed.” Using its ‘target-backwards’ problem-solving methodology, Spartan’s SSP portfolio aims to streamline the sterilization, inventory, and set-up costs of preparing an OR for surgery. Mr. Proffitt continued, “improving this broader system is not only the greatest opportunity to improve surgical outcomes but also one of the best avenues to reduce the cost of surgical care.”
Single-Use Sterile Instruments Reduce OR Time and Saves Costs
As a key part of its SSP portfolio development, Spartan worked directly with VA and DoD surgeons across the spectrum, from routine procedures to battlefield trauma. The endgame was to build pre-sterilized convenience kits containing the optimal mix of instruments needed for both microsurgical and minorsurgical procedures for the OR, clinic, as well as forward operating locations. Spartan developed customized convenience kits from scratch, based on direct surgeon feedback, in less than 120-days from concept to commercialization. Proffitt added, “We are a solutions-oriented company, and once we understand the surgeon preference, we will source the necessary items to meet the clinical need … and if we can’t find it, we’ll make it.”
Spartan’s SSP portfolio helps to reduce sterile processing delays, limits the impact of staff shortages in sterile processing departments, minimizes redundant capital purchases, and ensures required instruments are sterile and ready for use, on time, every time. Single-use instruments can save up to 30 minutes of total OR time,i reduce OR set-up time by 30%,ii and avoid frequent delays in surgery due to reusable instrument unavailability.iii
Single-use sterile instruments’ efficiency improvements can dramatically increase OR utilizationiv and patient throughput, facilitating an extra procedure in up to 51% of days.v They also result in direct cost savings — depending on the surgery type, the avoided staff time and sterilization requirements can save hundredsvi to well over a thousand dollarsvii per procedure.
Single-Use Instruments Actually Reduce Environmental Impact
It is commonly — mistakenly — understood that sterile, pre-packaged instruments generate a greater environmental impact. In fact, when considering the full sterilization lifecycle and disposable plastic wrapping used to maintain sterility of each reusable metal tray, a published study found, “the environmental impact of [single-use sterile instruments] was significantly lower” than reusable instruments.viii
Spartan Medical is excited to offer this innovative portfolio of single-use, sterile, pre-packaged surgical instruments as part of its mission to provide the world’s finest medical products to our nation—particularly to our soldiers, veterans, and their families. All products and solutions referenced are currently available on contract at VA and DoD medical centers across the country and overseas.