SpineX
SpineX Inc. today announced groundbreaking results in its first in-human study treating an adult with Cerebral Palsy (CP). The study, published in the esteemed medical journal BioElectronic Medicine, demonstrates significant functional improvements in an adult with CP after treatment with the company’s proprietary non-surgical SCiP™ therapy.
Led by Rahul Sachdeva, PhD and Kristin Girshin, PT, DPT
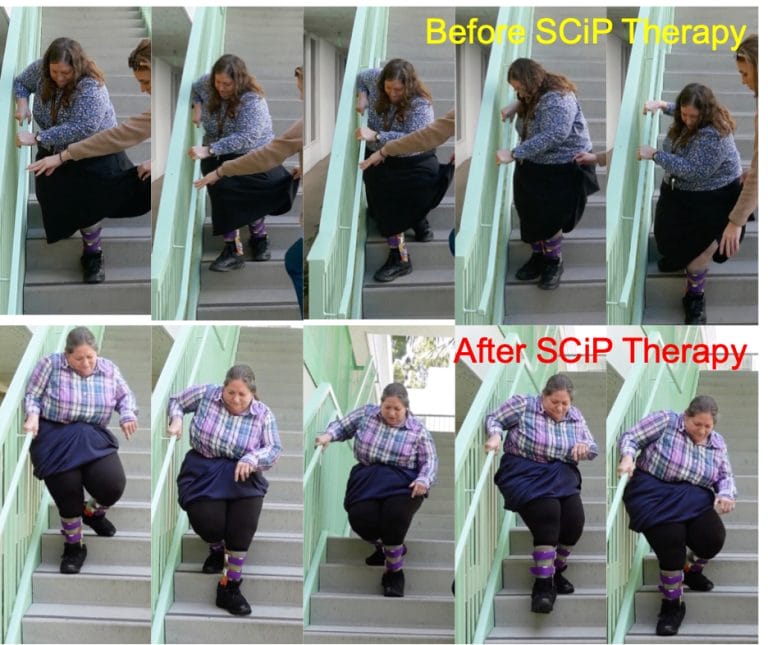
This study affirms how after just eight weeks of SCiP therapy treatment, a 60-year-old woman living with CP is able to perform daily living activities with minimal external assistance. “This is an absolute game changer for the CP community,” says the patient, who has lived with CP her entire life. Despite living with this severely debilitating condition, she has managed her symptoms with current standard of care therapies. However, she consistently encountered limited mobility and spent a great deal of energy completing routine tasks.
“After eight weeks of SCiP therapy, my balance was better, I was more confident in walking and climbing stairs with minimal assistance and was able to get dressed in less than half my usual time,” she says. “Over the years, I’ve tried every possible therapy available, but none of them had the impact that SCiP had, and in such a short period of time.”
Breakthrough Device Designation
SpineX has Breakthrough Device Designation (BDD) from the US FDA for SCiP and its proposed treatment of CP. SpineX has also engaged with the FDA to align on a proposed clinical trial to be conducted later this year, with the results expected to lead to FDA clearance of the SCiP device for the treatment of CP. “SCiP is a key missing piece in the puzzle for the treatment of CP,” says Dr. Girshin, lead therapist for the study and a key opinion leader within the CP community. “I am excited by the potential impact that SCiP could have on the CP population for both adults and children.”
About SpineX Inc.
SpineX Inc. is a clinical stage bioelectric MedTech company developing noninvasive spinal cord neuromodulation devices as a platform technology. SCONE™ and SCiP™ are two FDA-designated Breakthrough Devices being developed by SpineX for the treatment of adults with Neurogenic Bladder and Children with Cerebral Palsy respectively. SCONE™ and SCiP™ are investigational devices and are limited by Federal (USA) law to investigational use only. FDA has not yet reviewed the safety and effectiveness of SCONE™ and SCiP™.