In the process of testing the world’s first aerosol-safe non-invasive ventilation (NIV) mask, a freelance scientist from open talent platform Kolabtree has supported Israeli medical device manufacturer Inspir Labs. The freelancer, Dr. Nicholas Miceli, carried out a statistical analysis comparing the infection risk for those wearing the new mask against competitive products. Inspir Labs used this analysis to better understand the implications of different silicone and polycarbonate types, mask-face dynamics, and more.
Aerosol-generating procedures (AGPs) create large amounts of small droplets that remain buoyant in the air, which carry a severe risk of infection for patients and healthcare workers. Unlike respiratory droplets, aerosols are small enough to penetrate or circumnavigate most surgical masks. At the start of the pandemic, this risk meant that many hospitals refrained from giving patients non-invasive ventilation (NIV) for fear of infection, leading to more intubations as a result. To combat this, Labs designed an NIV mask that uses a pressure gradient to prevent aerosol emissions from being released into the environment.
“We saw how the pandemic exacerbated the risk caused by aerosol hazards — developing NIV technology that takes into consideration not only the patient, but also the medical staff was the obvious solution,” explained Nadav Nahmias, Chief Technology Officer at Inspir Labs. “During product development, we needed to compare the infection risk for various common viruses in both non-vented and medium-vented hospital wards to see how effective our mask would be. We needed to quantify this information so that we could analyze and present it when applying for regulatory approvals and presenting it to physicians.”
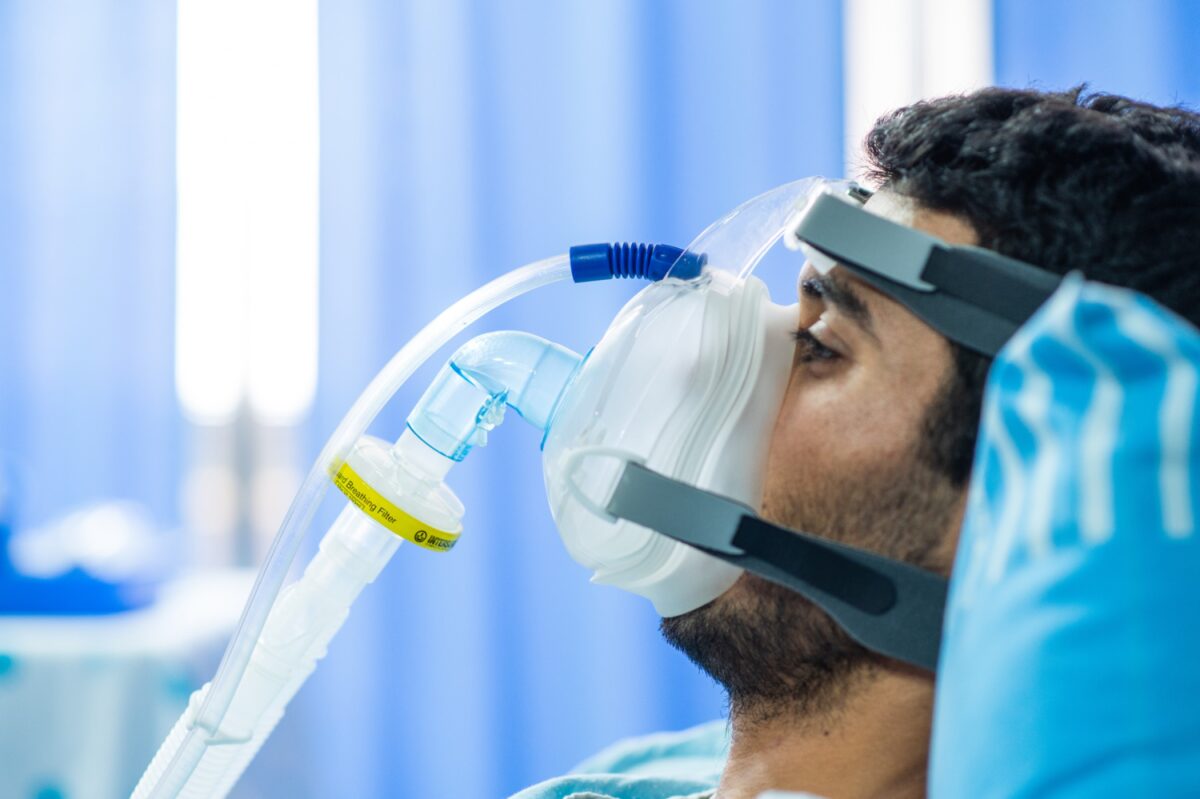
Inspir Labs posted the project on Kolabtree and was contacted by Dr. Nicholas Miceli, a freelance epidemiology expert. An associate professor of human resource management and healthcare management at Park University in Missouri, Nicholas was previously a researcher at the Ohio Department of Health and had also taught statistics and finite mathematics. This experience provided Nicholas with the necessary skills to carry out a statistical analysis for Inspir Labs, comparing the performance of the new aerosol-safe mask with competitive products.
“Inspir Labs provided me with a journal article that used a specific analytical method and requested that I replicate the analysis with the data provided,” explained Dr Miceli. “Working together with a colleague from Park University’s physics department, I found that the client’s mask performed better than the competition’s.”
Following this important statistical analysis, Inspir Labs progressed into sales processes and regulatory approvals. The mask has now received regulatory approval in Canada, Japan and Israel and is being used as an infection control device in multiple hospitals, keeping healthcare workers safe during AGPs.
Need a freelancer for your medical device project? Post your project for free https://www.kolabtree.com/create-project.