With the explosive growth in minimally invasive surgery (MIS) that began more than two decades ago, researchers and surgeons rapidly saw the potential to perform many procedures using less invasive approaches that could reduce both trauma and scarring without compromising quality and patient outcomes. In fact, in many cases MIS has been shown to help improve clinical outcomes and lower costs, with less damage to healthy tissue, shorter recovery times and reduced risk of complications and patient compromise. Laparoscopic surgery, the most common and earliest form of MIS, is a specialized technique that is now widely used for gall bladder and gynecologic surgeries as well as many gastro-intestinal surgeries.
As an adjunct to adoption of MIS options, surgeons and advanced endoscopists in recent years have also worked to develop strategies to perform many different procedures by targeting disease sites via the natural endoluminal cavities in the body, including the mouth, rectum, and vagina. Known as “endoluminal surgery,” this approach positions doctors to perform a range of procedures through a natural body cavity, making is possible to initiate surgical procedures closer to the site of disease in the body. By taking advantage of access via natural cavities or orifices of the body, endoluminal surgery also presents several other advantages including fewer external incisions, which typically result in visible scarring, minimal disruption of healthy tissue, and reduced patient trauma even beyond what is achievable through conventional MIS. Within just the past few years, innovative surgeons and advanced endoscopists around the world have recognized these benefits and have explored techniques in endoluminal surgery.
While endoluminal surgery has many clear advantages, a key goal and a considerable challenge has been the ability to access a target location in the body with all the surgical instruments necessary to perform a procedure. For example, as seen with endoluminal surgery, endoscopists may use a colonoscope or an esophagogastroduodenoscopy (EGD) scope to perform therapeutic interventions, but treatment can be limited due to available technology that requires them to perform procedures using only one very specialized instrument at a time. Recently, the team at EndoQuest Robotics™ has made significant progress in addressing this challenge by developing a robotic system for endoluminal MIS that allows physicians to perform procedures as they would in traditional open or laparoscopic surgeries – making it possible for surgeons to use up to three instruments simultaneously and implement optimal surgical techniques. This advancement significantly expands the potential to consider endoluminal robotic surgery for a broader range of clinical procedures in the years ahead.
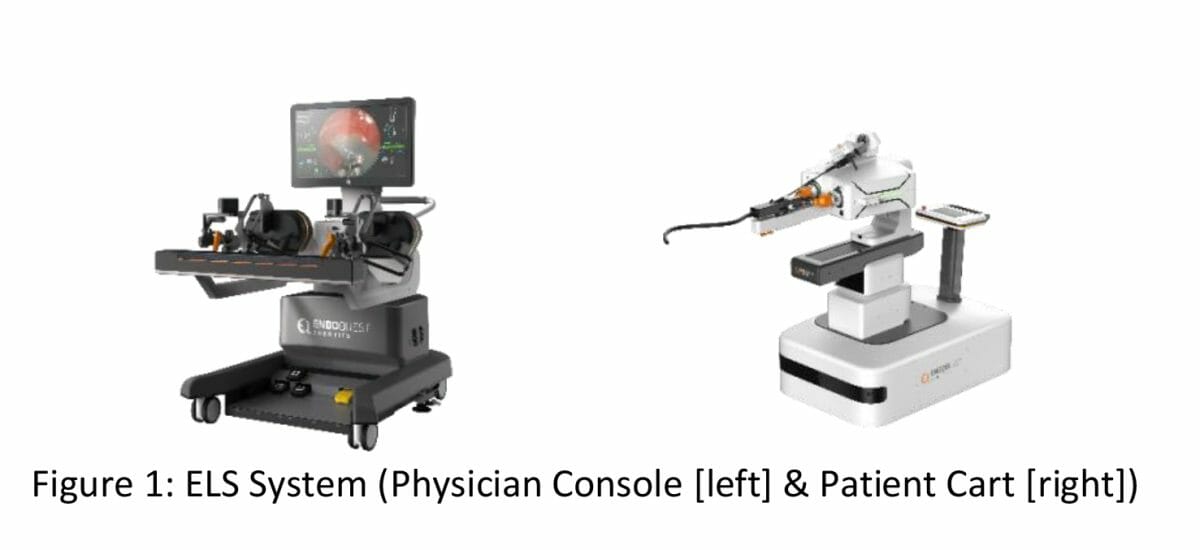
The EndoQuest Robotics Endoluminal Surgical (ELS) System is uniquely designed to accommodate conventional surgical techniques including triangulation, traction, countertraction, and two-handed suturing. The system consists of the Physician Console and Patient Cart (Figure 1), which together enable highly precise robotic-assisted control of the ELS instruments and of the Overtube and Videoscope. The flexible and steerable Overtube can accommodate simultaneous access to the target for two surgical instruments in addition to the Videoscope. Additional channels can also support needs in insufflation (blowing a substance such as a gas into a body cavity to create working space), suction and irrigation, as well as access to the site for third-party endoscopic devices. The fully articulating robotic instruments have seven degrees of freedom, providing physicians with the levels of dexterity, precision and flexibility that are required to perform endoluminal surgeries safely and effectively. The technology supports surgical techniques and maneuvers that are not achievable in conventional endoscopy.
Depending on the natural orifice used to access the site of disease, the ELS System makes it possible to expand the clinical options in endoluminal access and perform an ever-broader range of procedures. For example, transanal access could be an option for polyp resections and total rectal lesion resections whereas transoral access has potential applications in bariatric surgery, gastric and esophageal lesion resections, fundoplication, myotomy, tonsillectomy, pharyngectomy, and laryngectomy. The system may also support transvaginal access for a range of needs in gynecology including hysterectomy, oophorectomy, and salpingectomy.
In addition to these potential future applications, another aim in robotic endoluminal surgery is reduction in risk of complications, which many physicians believe could exceed the reductions achieved with the transition from open to laparoscopic procedures. For many patients, less invasive procedures with faster recovery times can also potentially reduce healthcare costs. In just one example, tens of thousands of patients are treated with partial colectomies each year that could be potentially avoided if physicians were able to treat these lesions using the ELS System.
As the development of the ELS System proceeds toward regulatory review, some leading physicians and hospital systems are moving ahead with plans to install the technology now to remain ahead of the curve in terms of research and innovation. In August 2022, EndoQuest Robotics announced that Brigham & Women’s Hospital in Boston, Massachusetts, will be the first U.S. medical facility to use the ELS System for research and clinical development.
According to Christopher C. Thompson, MD, Professor of Medicine and Director of Endoscopy, Brigham and Women’s Hospital, “Successful endoluminal surgery requires innovation in technology and procedural techniques. The EndoQuest System is the world’s first fully robotic platform specifically designed for gastrointestinal tract surgery and can enable endoscopists to perform many procedures that are very challenging or not possible today.”
With the ELS system, the EndoQuest team developed surgery enabling technology that solves many of the clinical challenges facing a wide range of MIS specialties. By empowering the system with both the flexibility of endoscopy and instruments that allow for conventional two-handed surgical technique, doctors will have new capabilities that will advance current techniques, close the prohibitive technologic gaps of the past, and provide for scar-free procedures.
EndoQuest’s ELS System is under development, has not been cleared by the U.S. Food and Drug Administration (FDA) and is not for commercial sale in the United States.
