POINTek, Inc., a global leader and provider of high performance high-end Athermal AWGs, announced the launching of new Ultra Low Loss Athermal AWG (ULL-AAWG) Multiplexer/Demultiplexer product which is shown in Figure 1.

“The Gaussian ULL-AAWG achieves ultra-low insertion loss of typically -1.5dB at the ±12.5GHz passband window. This is 2dB better than standard Gaussian AAWGs in the market. The Flat-Top LL-AAWG achieves also low insertion loss of typically -3.5dB at the ±12.5GHz passband window, offering 2dB better than standard Flat-top AAWGs in the market,” says Dr. T.H. Rhee, CEO of POINTek. “We upgraded the currently available Gaussian and Flat-top AAWGs to meet customer’s unique operational environment optimally by improving insertion loss one more level upwards. Our new ultra-low loss ULL-AAWG products are available as AAWG bricks, Standard 1RU chassis and LGX cassettes,” Rhee adds.
“Our ultra low insertion loss Athermal AWG Multiplexers along with temperature-hardened low-drift AAWG specifically designed for the industrial temperature applications will be marketed aggressively through partnership companies in the East Asia and EU regions after its success in the North America,” says Dr. Donald Yu, CMO of POINTek, who runs global marketing operations from Los Angeles, California.
Figure 2 illustrates spectral differences between ultra-low loss AAWGs versus Standard AAWGs.
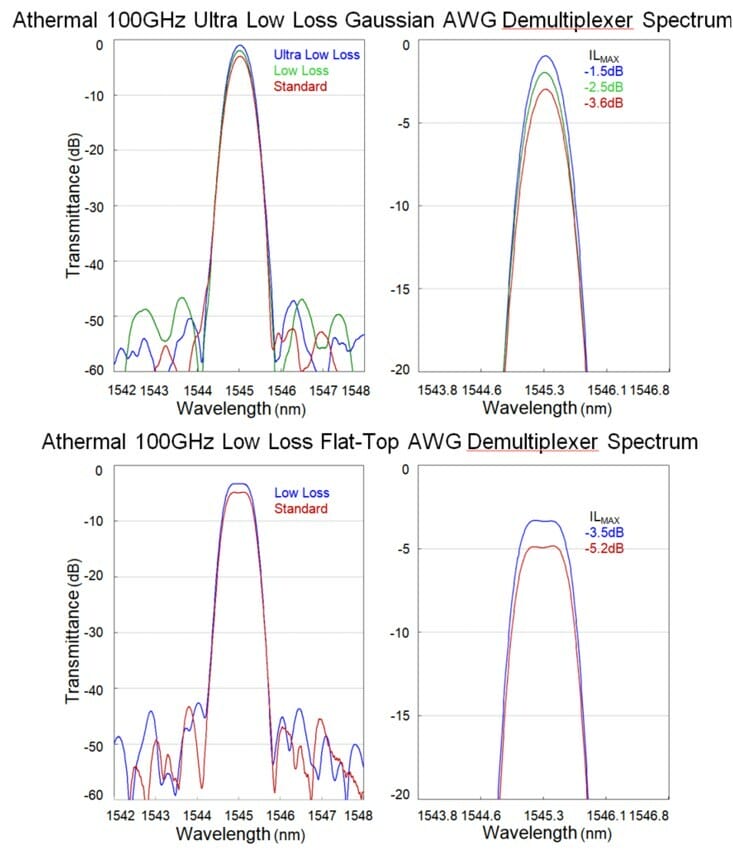
Figure 2. (Top) Gaussian 100GHz Ultra Low Loss AAWG Demultiplexer Optical Spectrum: These figures depict the insertion loss value of -1.5dB at ±12.5GHz passband window for ULL Gaussian AAWG; (Bottom) Flat-Top 100GHz Low Loss AAWG Demultiplexer Optical Spectrum: These figures depict the insertion loss value of -3.5dB at ±12.5GHz passband window for LL Flat-Top AAWG.
“POINTek’s new Ultra Low-Loss Athermal AWGs will stand to be appealing to today’s high-end Data Center and Mobile Fronthaul applications where the IL margin is quite important. The new Ultra Low Loss AWGs will be displayed at the coming OFC2024 Conference exhibit floor, booth 4845,” Yu says.
According to Yu, POINTek’s AAWG are 100% passive, 100% TAA compliant, and individually customized. POINTek’s two decades-long seasoned optical packaging technique not only enables the company to manufacture all types of customized high-end discriminator AAWGs but also allows to refine its athermalization technology one level higher than that of commercial market level. POINTek’s R&D Team has released AAWG product innovations, judiciously demonstrating POINT’s commitment to continual improvement to AAWGs and its integrated passive optical device technology.
“We are selling not only Low Loss AAWGs, but also selling other high-performance AAWGs adequate for the customer-specific applications. POINTek has since January 2004 manufactured and marketed globally its own athermalization IP-based highly reliable top-end AAWG products in all types of spectral characteristics, configurations, and channels in various customer-specific form factors. We will also present our recent products including AAWGs for the customer–specific applications, such as Standard Chassis and Cassettes, 5G Wireless Fronthaul, Industrial Temperature, High Density and Small Form Factor, Channel Monitor and Sensors, and Silicon Phonics Fiber Arrays, at the OFC2024 Conference” Yu adds.

