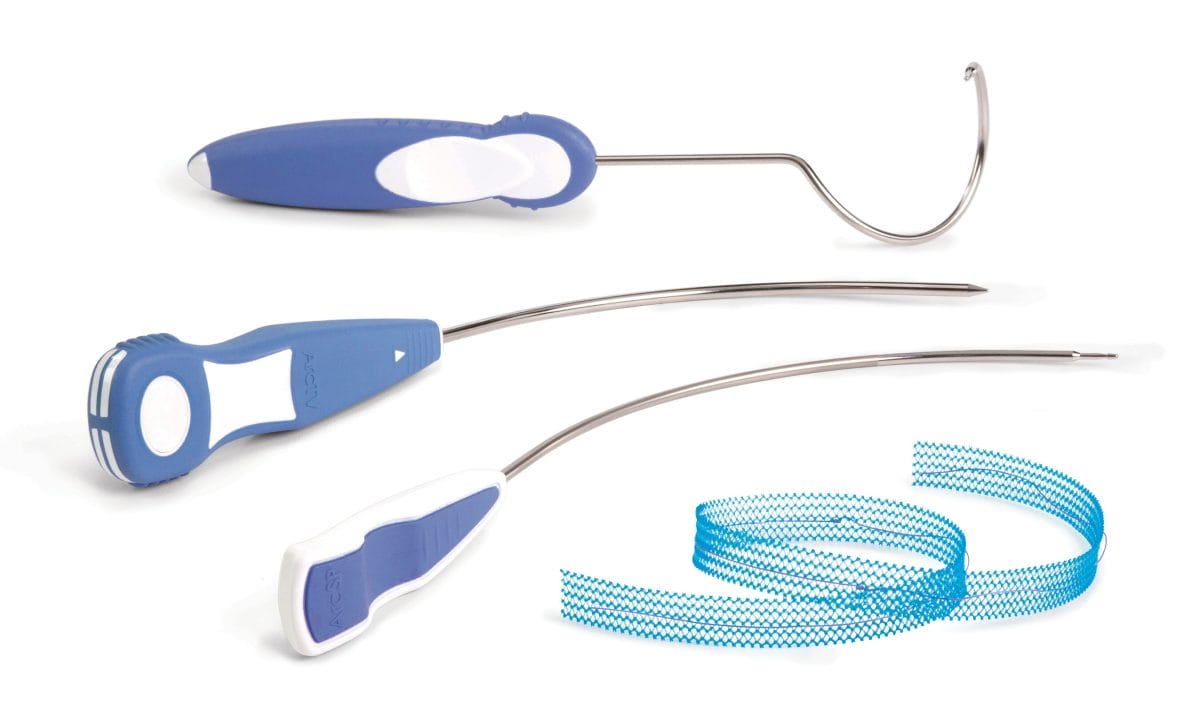
ArcSP™ Suprapubic Sling System and the ArcTO™ Transobturator Sling System
UroCure and LiNA Medical USA are pleased to announce the nationwide launch of UroCure’s two latest innovations of surgical solutions for female stress urinary incontinence: the ArcSP™ Suprapubic Sling System and the ArcTO™ Transobturator Sling System.
UroCure ArcTV® Transvaginal Sling
These two additions complement the current UroCure ArcTV® Transvaginal Sling on the market. ArcSP will now provide a top-down, retropubic approach and ArcTO will accommodate surgeons that prefer an outside-in, transobturator approach. All three systems are based on AMS’ best-in-class slings, while incorporating UroCure’s laser cut sling with its patented stabilizing suture. The absorbable stabilizing suture is a key innovation of UroCure’s sling designed to maintain its open pore structure and to protect the sling from deformation during placement, tensioning, and sheath removal.
John Nealon, CEO of UroCure
“At UroCure, our mission has always been to empower patients and physicians with innovative solutions. The nationwide launch of ArcTO and ArcSP represents a significant milestone in our commitment to expand our product offerings and offer cutting-edge solutions for the treatment of stress urinary incontinence.”
Collaboration with LiNA Medical USA
UroCure’s collaboration with LiNA Medical USA, further amplifies the reach and impact of these additional slings. Lars Melbye, President of LiNA Medical USA shared his enthusiasm for the partnership, saying, “We are thrilled to continue our partnership with UroCure by introducing the ArcTO and ArcSP to the U.S. market nationwide. LiNA Medical USA has seen the immense value the ArcTV sling system adds for surgeons and their patients. Now offering three sling solutions will be an impactful offering nationwide.”
More Information
For more information about UroCure’s Arc sling systems portfolio, please visit UroCure’s website https://urocure.com or contact LiNA Medical to connect with your local sales representative (info@linamed.com, 855-546-2633).