Mobile health technology company UTM:Healthcare today announced that it has enhanced the connectivity of its remote patient monitoring (RPM) solution through the use of new biometric devices that have greater data transmission capabilities.
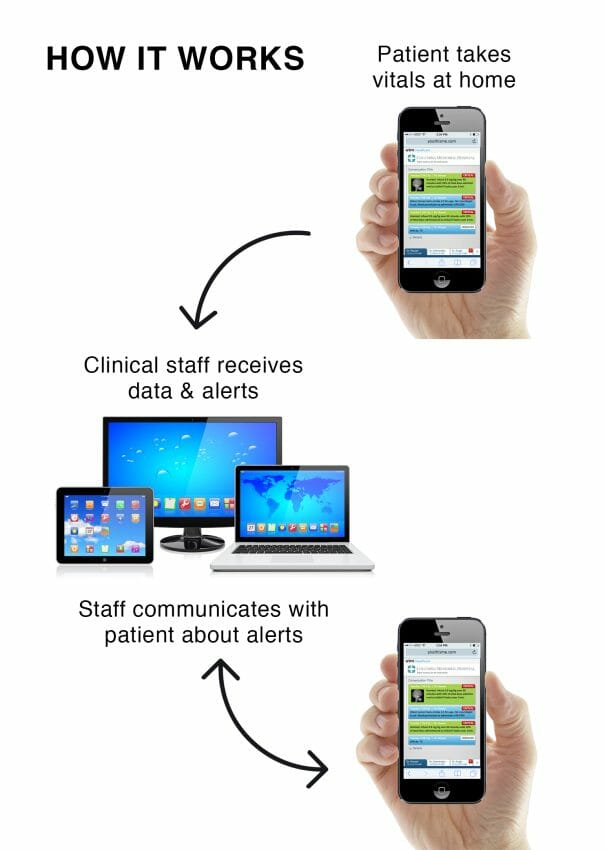
UTM:Healthcare has advanced its UTM:RPM solution to make it compatible with cellular-enabled blood pressure monitors, blood glucose meters and pulse oximetry devices. The technology is ideal for monitoring the health of patients who don’t use or can’t afford a smartphone, or may have difficulty pairing biometric devices with their mobile phones.
“With this innovation we are making it easier for even more of the patient population to use remote monitoring technology so that they can continue to maintain their health at home while staying in regular contact with their medical team,” said Seth Lachterman, a partner and co-founder of UTM:Healthcare and its parent company, YouThisMe.
The UTM:RPM solution helps clinicians in various health care settings to remotely monitor their patients’ common chronic conditions, including congestive heart failure, COPD, diabetes and pneumonia. With cellular-enabled biometric devices, patients can take readings of health data at home and transmit directly to the UTM:RPM system, with or without the assistance of the RPM app.
“We look forward to collaborating with health care providers to implement telemedicine tools to improve health outcomes for patients,” Lachterman said.
