Vessi Medical announced that it successfully completed a first-in-human case with its minimally invasive cryoablation solution for superficial bladder cancer (NMIBC). The procedure was performed at Rambam Healthcare Campus in Haifa, Israel.
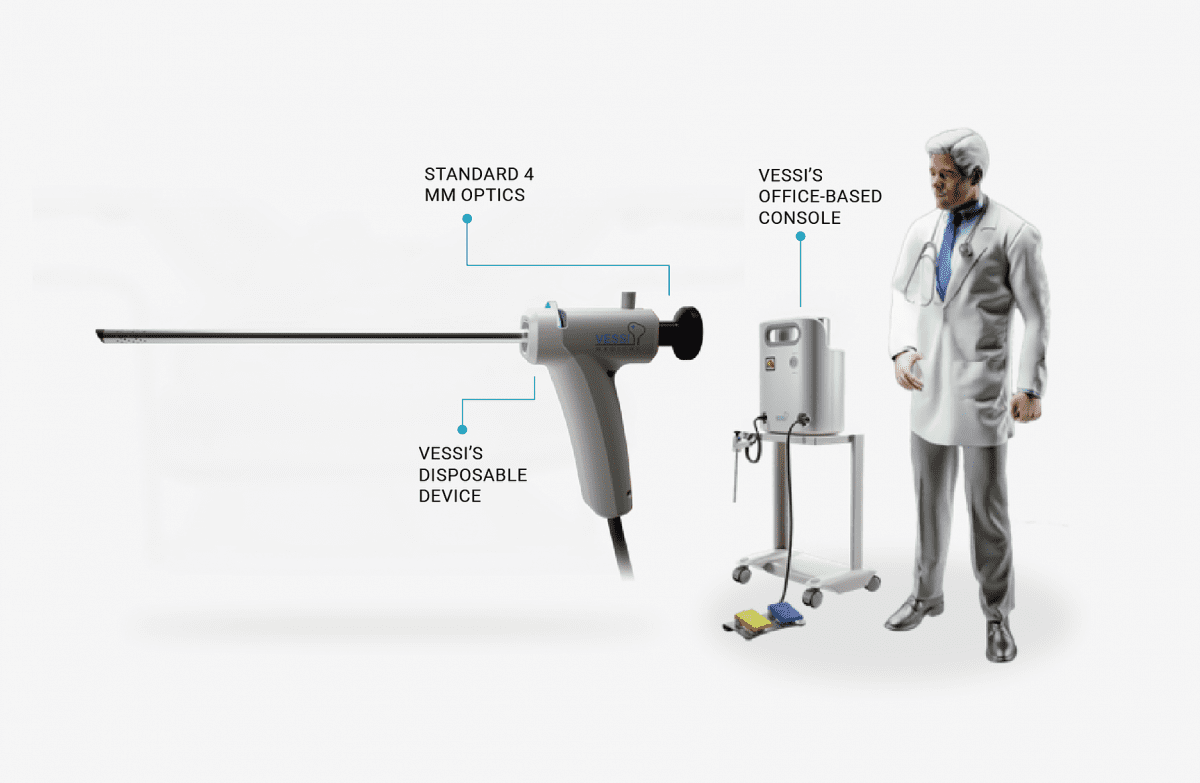
The procedure demonstrated ease of use, speed of activation and completion, without complications or bleeding. Additionally, clear inner bladder visualization and good tumor reaction were recorded. The patient was discharged the following day and reported no pain. Follow-up will continue over the coming weeks. The first patient procedure marks the beginning of the Vessi Medical first-in-human study, which will support product development towards the regulatory process.
Vessi Medical aims to provide an alternative to current first-line treatment, Transurethral Resection of Bladder Tumor (TURBT), a costly and invasive surgical procedure, performed under general anesthesia, with up to 60% recurrence of cancer, and many complications, resulting in poor quality of life. Vessi’s minimally invasive bladder-specific cryotherapy technology offers a new therapy alternative to surgery and is expected to improve quality of life for many NMIBC patients by eliminating the costly and problematic issues experienced with current treatments.
Dr. Azik Hoffman, Attending Physician, Department of Urology, Rambam, reported: “I’m proud to have performed the first procedure with Vessi’s system, which worked like a simple cystoscopy, translating to a short learning curve. For the selected lesion, response to cryotherapy was as expected, without any bleeding, and visibility was excellent. Performing cryotherapy for bladder papillary lesions using minimal sedation seems a tangible possibility given the right setup.”
Prof. Gilad E. Amiel, Department Chair of Urology, Rambam Healthcare Campus, and scientific advisor for the company adds: “The cryo procedure demonstrated an immediate change in the three-dimensional structure of the tumor. The freezing of the lesion was easily noticeable and it froze completely in only a few seconds, with similar quick thawing. We performed two cycles of freezing and thawing, to ensure apoptosis and necrosis of the lesion. We look forward to being able to offer cryotherapy for bladder papillary lesions as an office procedure without anesthesia, in the future.”
Vessi Medical CEO Eyal Kochavi remarked, “Demonstrating the technology in humans is a giant step forward towards making cryo-spray ablation a significant tool for the treatment of superficial bladder cancer and, in the future, for other bladder diseases such as overactive bladder.”
