Virtual Incision Corporation, a medical device company pioneering the world’s first miniaturized robotic-assisted surgery (RAS) platform, today announced the U.S. Food and Drug Administration (FDA) has approved an Investigational Device Exemption (IDE) supplement to complete the final stage of its clinical study analyzing the MIRA® Platform in bowel resection procedures. The approval was supported by a favorable interim clinical study report on the safety profile of MIRA.
The IDE supplement approval puts Virtual Incision on track to obtain the clinical evidence needed to bring innovation to the soft tissue surgical robotics industry, a market that has been historically dominated by a single player. Results of the completed study will support MIRA’s upcoming FDA De Novo application for market authorization.
The first cases of the study were completed at Bryan Medical Center in Lincoln, Neb. by Dr. Michael Jobst and Dr. Kelly Krier, and at Lankenau Medical Center in Wynnewood, Pa. by Dr. John Marks and Dr. Henry Schoonyoung.
“Our clinical experience has been extremely positive so far,” said Dr. Jobst, the first surgeon in the world to operate with the device. “I was able to perform 100% of the dissection with MIRA in all of my cases. We have also been pleased with its accessibility and efficiency. I operated on eight patients in five different operating rooms, and that’s something that’s just not possible with mainframe RAS platforms. MIRA has the potential to bring the benefits of minimally invasive surgery to more patients, and that’s truly exciting.”
“MIRA was created to address the limitations of traditional robotic-assisted mainframe machines. We miniaturized and simplified MIRA to make it more accessible, easy to use, and easy to adopt,” said John Murphy, president and chief executive officer of Virtual Incision. “These are the features that will allow surgeons to treat more patients each day. It is encouraging to see MIRA demonstrating the potential to help surgeons perform simplified robotic procedures safely and precisely. Completing the final stage of our clinical study will be a key milestone along MIRA’s regulatory pathway, and we will continue to focus on clinical excellence to best support the innovation we provide to patients and surgeons.”
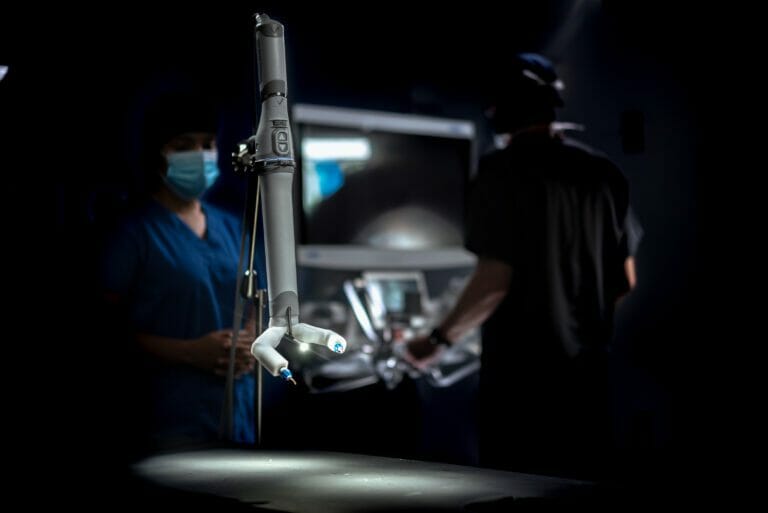
About the MIRA® Platform
Virtual Incision’s MIRA Platform is the world’s first miniaturized robotic-assisted surgery (RAS) platform. Its small, sleek design is planned to offer the benefits of RAS during abdominal procedures without the logistical inefficiencies of traditional mainframe robotics. The easily accessible device weighs only two pounds and can be used in any operating room — a dedicated mainframe room is unnecessary. With its drape- and dock- free design and portability, MIRA is quick to set up, clean up, and move in between cases, enabling an increased robotic-assisted surgery caseload. With MIRA, every operating room is robot-ready.