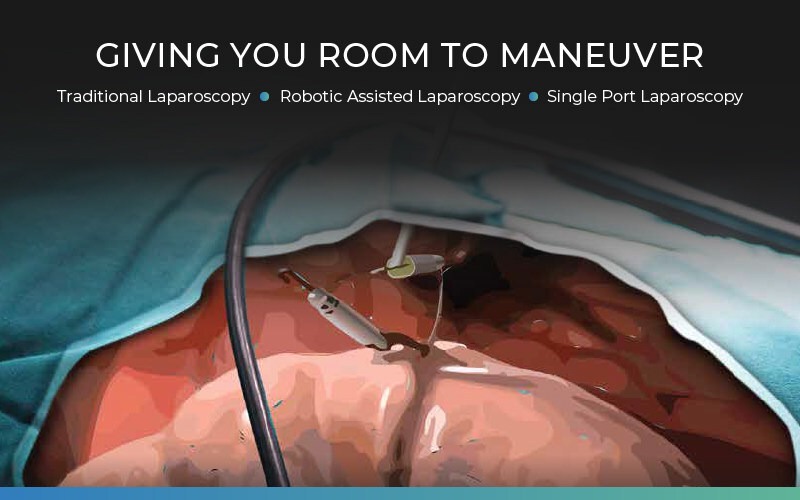
Virtual-Ports Ltd is thrilled to announce the attainment of CE certification under the new MDR (Medical Device Regulation 2017/745) for its groundbreaking surgical products. Renowned for their single-hand, multi-application design, Virtual-Ports’ solutions are set to revolutionize abdominal surgery by significantly improving visibility and access within the surgical space.
At the core of Virtual-Ports’ innovation is its MicroAnchoring™ technology, a proprietary system engineered to empower surgeons with enhanced control and clinical precision during laparoscopic procedures. This advancement not only reduces access ports and reliance on operating room assistance but also eliminates the need for a fourth robotic arm in Robotic-assisted surgery, thus mitigating complications and related costs. The acquisition of CE certification is crucial for commercial product sales in European, UK, and other international markets that recognize CE marks.
CEO Yuval Yaskil of Virtual-Ports Remarked
“Attaining the CE certification under the new MDR requirements not only allows Virtual-Ports to access a long-awaited multi-billion-dollar market, but it is also a testament to the quality and advancement of Virtual-Ports as a company. This milestone underscores our steadfast commitment to empowering surgeons and healthcare systems with cutting-edge solutions that promote safer and more cost-effective clinical outcomes.”
With the CE certification in hand, Virtual-Ports is poised to introduce its transformative technology to medical professionals and patients across Europe, further cementing its position as a leader in the surgical technology landscape.