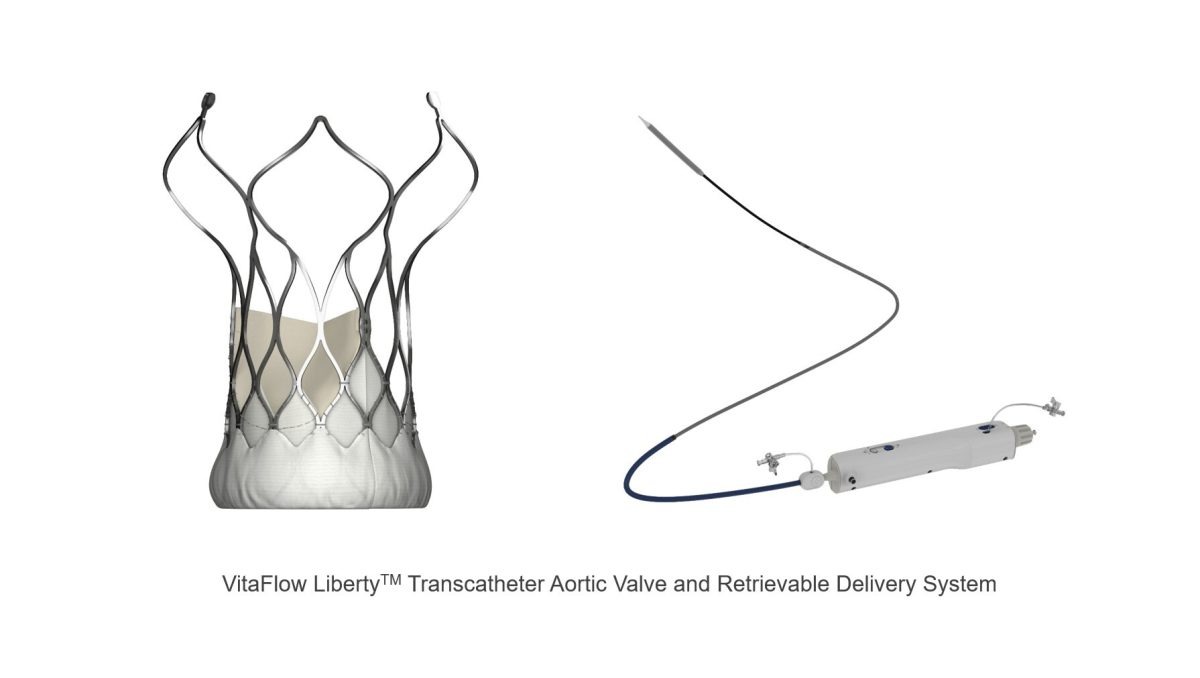
MicroPort® CardioFlow Medtech Corporation (CardioFlow) (Stock Code: 02160.HK) recently announced that its self-developed second-generation transcatheter aortic valve implantation (TAVI) device, the VitaFlow LibertyTM Transcatheter Aortic Valve and Retrievable Delivery System (VitaFlow LibertyTM), has received EU CE-MDR certification. This certification highlights VitaFlow LibertyTM as a pioneering TAVI solution, that sets a new benchmark in transcatheter heart valve treatments.
With over 47 million patients globally[1] suffering from aortic valve stenosis and regurgitation, the prevalence rates of these conditions are on the rise due to an aging population. The TAVI solution provided by CardioFlow, which avoids open-heart surgery and offers various benefits like minimal trauma, quick recovery, and enhanced quality of life, is increasingly becoming a preferred choice for patients with aortic heart valve disease.
CardioFlow, one of the world’s leading innovative medical device companies, has entered the field of structural heart disease when the field was still at an early adoption phase. Originating and headquartered in Shanghai, China, CardioFlow was listed on the Hong Kong Stock Exchange on February 2021. The company has a diverse product pipeline resulting from independent and collaborative research, covering structural heart devices such as transcatheter aortic, mitral, and tricuspid valves, left atrial appendage occludes, and accessories. Leveraging its technological expertise and capacity for innovation, the company has successfully obtained approvals and launched several TAVI products globally, among which VitaFlow LibertyTM stands out as the world’s only electric retrievable transcatheter aortic valve system. The VitaFlowTM series TAVI solution along with its accessory – the AlwideTM series Balloon Catheter, has successfully covered nearly 700 core hospitals in 10 countries and regions, treating more than 10,000 patients with aortic valve disease worldwide.
The clinical data from VitaFlowTM series valves were revealed at PCR London Valves 2023, a leading global conference on structural heart diseases. These results highlight VitaFlowTM‘s exceptional long-term clinical performance aligning with international top-tier standards. The long-term results of VitaFlowTM in high surgical risk patients with severe aortic stenosis showed promising outcomes in all-cause mortality, cardiac mortality, and permanent pacemaker implantation rates for patients over seven years, compared to other similar studies. During the conference, Dr. Darren Mylotte from Galway University Hospitals, Ireland, commented on the excellent data, and introduced the advantages of VitaFlow LibertyTM in its one of a kind motorized delivery system. The system can assist the valve to position easily due to its flexibility and 360° range of motion when treating complex anatomical patients with severe angled aortic arch deformities. The valve can also be fully retrieved and repositioned when released to 75%, and provides up to 3 retrievable opportunities for each procedure, thereby further optimizing the implantation effect. Additionally, it can effectively ensure the stability of valve release, reduce valve displacement, and make the procedure more controllable.
Before launching into the EU market, VitaFlow LibertyTM conducted pre-market clinical implantations at Galway University Hospital in Ireland, Rigshospitalet (Copenhagen University Hospital) in Denmark, and St Thomas’ Hospital as well as Brighton & Sussex University Hospitals NHS Trust in the United Kingdom, and received very high appraisal from many well-known clinical professionals. Dr. Ole De Backer, a professor of interventional cardiology, who led the TAVI procedures at Rigshospitalet stated, “The overall release process of VitaFlow LibertyTM is notably stable, ensuring precise positioning. This stability is especially crucial in patients with a small left ventricles, where VitaFlow LibertyTM consistently achieves stable and precise deployment, fully demonstrating its distinct advantages. We look forward to its positive impact on a broader patient population following CE certification.” It has been reported that the European post-market clinical project will also be planned to start this year.
As part of CardioFlow’s global expansion roadmap, the company has also achieved significant milestones with CE application on three of its products, including the AlwideTM Plus Balloon Catheter, an essential accessory for aortic valve procedures, as well as the AnchorManTM Left Atrial Appendage Closure System and the AnchorManTM Left Atrial Appendage Access System, both developed by its subsidiary, CardioAdvent.
Jeff Lindstrom, President of CardioFlow, stated, “The certification of VitaFlow LibertyTM by the CE regulatory body under MDR, is a testament to CardioFlow’s world-class R&D, quality, and clinical capabilities. This recognition will expedite the global clinical adoption of the VitaFlowTM series along with other innovative products, advancing CardioFlow’s globalization strategy. This achievement also positions us to make a more substantial contributions to developments in the field of heart valve interventions, ultimately benefiting patients across the globe.”
Guoming Chen, Chairman of CardioFlow, commented, “Securing the EU CE-MDR marking for VitaFlow LibertyTM is not just a passport for the product’s entry into the European market, it also represents a significant milestone in CardioFlow’s history and global roadmap. This achievement will assist in diversifying the company’s sources of sales revenue and bolstering our overall competitiveness with a steadfast commitment to world-class product innovation.”
|
1. Frost & Sullivan’s statistics, 2021 |