In a ground-breaking collaboration, leading biotechnology companies and research organizations in UK and Canada are entering into a R&D project jointly funded by a £1.1 million grant from Innovate UK (part of UK Research and Innovation) and the National Research Council of Canada Industrial Research Assistance Program (NRC IRAP). The project is led by VVector Bio and joined by the NRC, Revvity and Abselion.
Cell and Gene Therapies hold immense promise for curing a myriad of diseases, yet their widespread adoption is impeded by manufacturing limitations. Current methods face hurdles such as low yields, poor scalability, high costs, and quality testing challenges. Traditional approaches have yielded minimal improvements, requiring a fresh perspective.
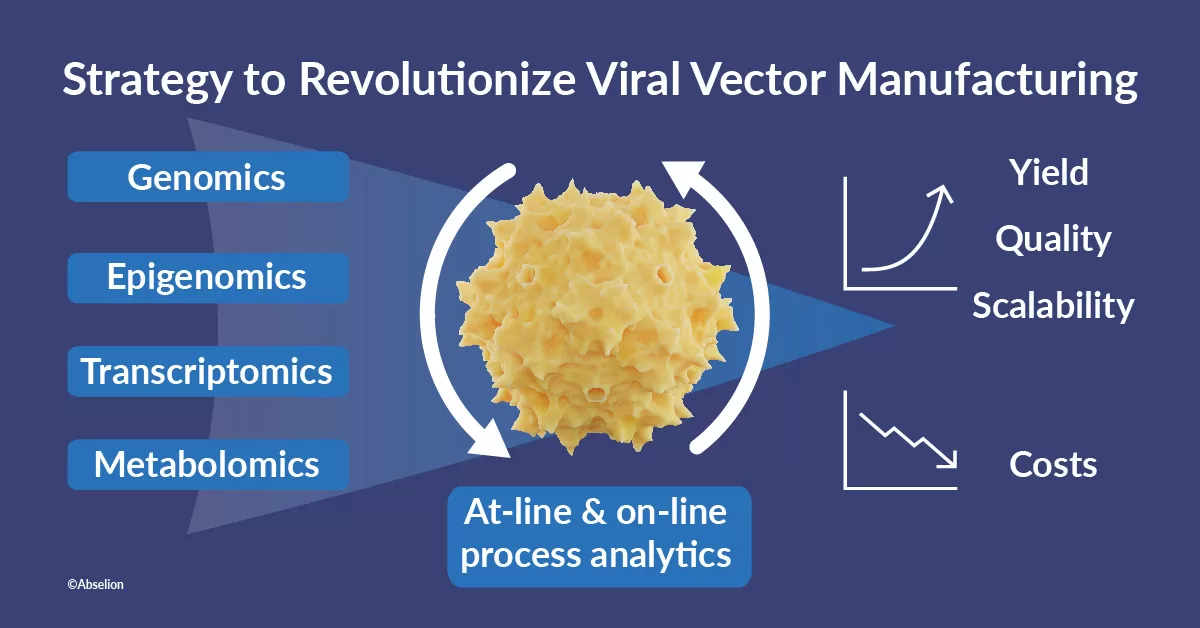
In response, the project consortium has devised a multifaceted strategy to revolutionize viral vector manufacturing. Central to this endeavour is the genetic modification of the widely used HEK-293 cell line. By meticulously analysing the cell line’s genome and transcriptome under producing and non-producing conditions, researchers aim to identify genes directly impacting viral vector yields and quality. Combined with an innovative approach to manufacturing and process monitoring empowered by on-line process analytics, the project seeks to disrupt the existing viral vector production process.
“This project represents a paradigm shift towards improving viral vector manufacturing yields,” remarked Alina Venereo Sanchez, CEO of VVector Bio. “By integrating genomics, epigenomics, transcriptomics, metabolomics and new ways of directly integrating analytics into production, we’re poised to unlock unprecedented production capabilities.”
The project builds upon NRC’s expertise in biomanufacturing and viral vector production. “Our past achievements underscore the feasibility of our approach, and we anticipate significant enhancements” emphasized Aziza Manceur at NRC.
“Revvity is excited to be part of this consortium” commented Dr. Alan Fletcher, Revvity’s Senior Vice President, Life Sciences. “Its goal aligns with our mission to help our customers overcome the challenges they face in accelerating the creation of next-generation therapies for patients. Together, we aim to drive innovation in the cell and gene manufacturing process, which will, in turn, help get life-changing therapies in the hands of patients that need them sooner.”
“Process optimization through at-line & on-line analytics is key to redefining viral vector manufacturing.” noted Ruizhi Wang, CEO of Abselion. “This project is the ultimate opportunity to showcase how it can accelerate development and result in more efficient and robust production of the next generation of therapeutics.”
The collaboration includes organizations from both Canada and the UK. It is funded as part of the “Canada-UK: Biomanufacturing of Biologics and Advanced Therapies” programme, which is jointly funded by Innovate UK and the National Research Council of Canada Industrial Research Assistance Program. The goal of the programme is to stimulate the development and implementation of innovative technologies in biomanufacturing.