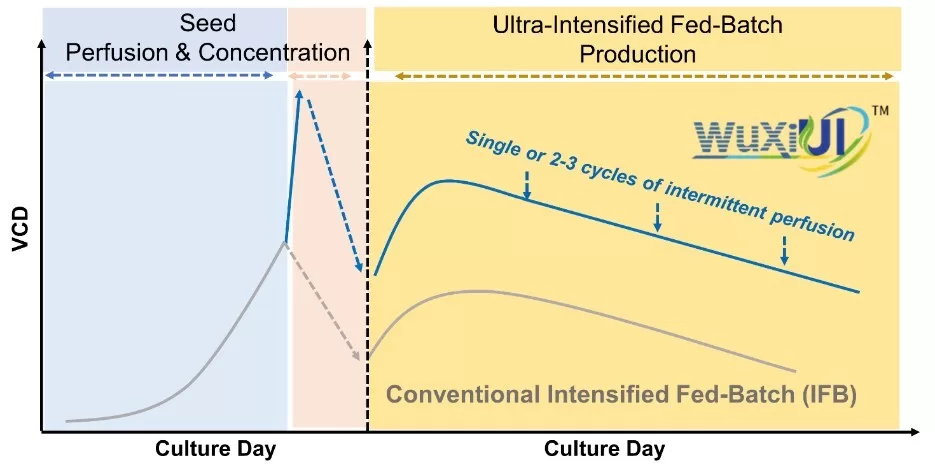
WuXi Biologics (“WuXi Bio”) (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), today announced that it has successfully accomplished 2,000L drug substance (DS) GMP manufacturing by utilizing its proprietary ultra-intensified fed-batch bioprocessing platform WuXiUI™. The platform achieved a titer of 18 g/L, a 4-fold increase compared to conventional fed-batch process, demonstrating the high efficiency of WuXiUI™ in improving productivity.
In addition, the company’s enhanced downstream technology platform enabled doubled purification processing capacity and similar impurity removal, which resulted in a 50% reduction in downstream processing time and a final DS yield of 70% with comparable product qualities. Moreover, WuXiUI™ downstream platform also enabled 30-50% reduction in the utilization of materials and consumables and therefore downsized waste generation. The improvement in total DS production contributed to significant reduction in manufacturing COGS, a substantial economic efficiency improvement in biologics production.
The WuXiUI™ platform, launched in 2023, enhances the productivity of multiple different CHO or other mammalian cell lines that express diverse product modalities, while maintaining desirable product qualities. It provides an effective solution for global clients to meet the growing demand for therapeutic proteins and antibodies with lower COGS. At the same time, it allows for a lower carbon footprint due to its more efficient media consumption, lower waste generation, and reduced demand for building space in the production line. The successful scale-up of the WuXiUI™ platform from bench scales to 2,000L GMP manufacturing confirms the robustness of the technology and its readiness for larger-scale production.
WuXiUI™’s strong performance was boosted through the application of MagniCHO™, WuXi Biologics’ proprietary platform cell culture media enriched with nutrients for intensified processes. Furthermore, operation efficiency and production robustness were improved by integrating the Raman Process Analytical Technology (PAT) tool into the scale-up manufacturing, not only for process monitoring, but also – for the first time under GMP settings – to provide real-time automated process control.
Dr. Chris Chen, CEO of WuXi Biologics, commented, “The successful application and achievement of WuXiUI™ is a result of our relentless pursuit of technology innovation to speed up biologics development while achieving cost efficiency for global clients. With this milestone, we have enhanced our capabilities to enable clients to bring more affordable, high-quality biologics to market, benefiting patients worldwide.”