- Coreline Soft Chosen as Exclusive AI Provider for France’s IMPULSION Lung Cancer Screening Pilot
- FastWave Medical Completes Coronary Laser IVL Study Enrollment, Secures 9th U.S. Patent
- Forget Textbooks – The 5 Ways E-Learning for Healthcare is Creating Smarter Doctors
- Pluri Partners with Miss Universe Skincare Introducing Exosome-Enhanced Regenerative Skincare
- Get Well, a leader in patient engagement software, and RhythmX AI, a leader in AI-powered precision care, have combined to form GW RhythmX to usher in the next generation of precision care
- Gallant Reports | FDA Completes Safety for Their First Ready-to-Use Stem Cell Therapy for Refractory FCGS
- Medicom Now Fully Contracted with 17 of 18 VA Regions, Announces New VISN 10 and 17 Agreements
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook

HeartBeam Advances Patient Convenience with At-Home ECG Technology
Growing clinical evidence supports the expanding role of mobile and home-based ECG capture in improving patient outcomes.
HeartBeam’s proprietary system offers a cable-free design that allows for high-fidelity ECG measurements without the complexity of conventional equipment and portable, credit card-sized design enables patients to record symptoms at home or wherever they are.
Recor Medical Announces Paradise Ultrasound Renal Denervation System Receives Manufacturing and Marketing Approval in Japan for the Treatment of Resistant Hypertension
his marks the first approval in the country for a medical device indicated for the disease
Owlet Achieves TGA Certification for Dream Sock™
Certification underscores Owlet’s commitment to bringing medically-certified infant monitoring technology to every family across the globe
Herculite Achieves 1,000 Days Without a Lost-Time Incident
This achievement highlights Herculite Associates’ commitment and vigilance in making safety a shared responsibility every single day.
Clinical Trials
Octapharma Releases Results for Phase 3 Superiority Study of Pediatric Acute-onset Neuropsychiatric Syndrome
Clinical Global Impression Data Showed Statistically Significant & Clinically Relevant Improvement in Pediatric PANS Patients.
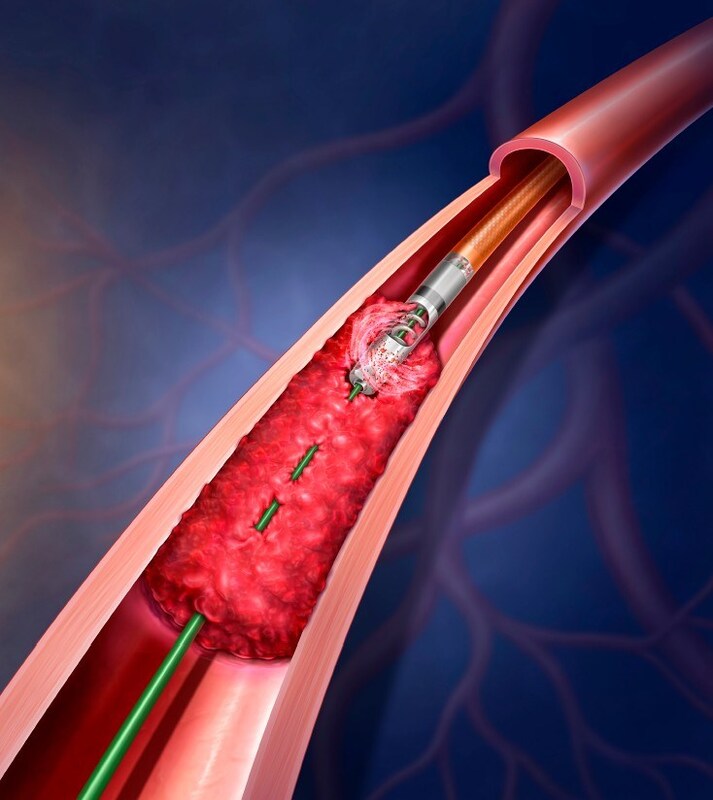
BD to Initiate Real-World Patient Data Registry for the Rotarex™ Atherectomy System in Treatment of Peripheral Artery Disease
Known as “XTRACT,” this prospective, multi-center, single-arm, post-market registry study will assess the clinical performance of the Rotarex™ Atherectomy System in the treatment of U.S. patients with PAD lesions.
Artificial Intelligence Tools Make Education Materials More Patient Friendly
“Our study shows that widely used large language models have the potential to transform patient education materials into more readable content, which is essential for patient empowerment and better health outcomes,” said Study senior author Jonah Feldman, MD, medical director of transformation and informatics at NYU Langone.
Biotechnology News
Neogap Therapeutics Selected for EIC Soft-landing Programme in Boston
The EIC Soft-landing Programme provides business development support and opportunities to connect with investors, academic institutions and life science stakeholders in Boston – a global hub for cancer research and biotechnology. Neogap’s combination of AI, bioinformatics and cell biology in developing personalised immunotherapy aligns well with the programme’s focus on deep tech companies.

BD Launches Landmark Cell Analyzer Featuring Breakthrough Spectral and Real-Time Cell Imaging Technologies
BD SpectralFX™ Technology maximizes the number of colors that can be used in flow cytometry, allowing scientists to analyze up to 50 or more characteristics of a single cell at one time, with optimal resolution and sensitivity, which is ideal for scientists pursuing novel biomarker discovery.
Piramal Pharma Ltd. Continues to Invest In USA; $90M in Expansions of Two US Sites
Addition of commercial-scale sterile injectables capabilities in Lexington, KY.
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

Holiday Stress & Heart Health: How AI-Powered Remote Cardiac Monitoring Redefines Cardiovascular Wellness During High-Stress Seasons | By Stuart Long, CEO, InfoBionic.Ai
In this article, Stuart Long explores the critical connection between stress and heart health, and discuss how AI-powered remote cardiac monitoring can help high-risk individuals avoid preventable cardiac events, especially during the holiday season.

Avania — Building a MedTech Advisory & Clinical Development Partner | By: Jason Monteleone, Chief Executive Officer & President of Avania
Avania will evolve, invest, and pivot with the industry so it can achieve its mission of partnering with MedTech sponsors to bring life-changing technology to market, resulting in improved healthcare outcomes.

Harnessing the Power of the Heart and Mind: Overcoming Atrial Fibrillation and Launching a Business | By Jim Kaveney
Jim Kaveney writes: “In both entrepreneurship and managing AFib, I have learned that the heart and mind are not in opposition but work together in powerful harmony. Whether navigating the unpredictable rhythms of my heart or the rollercoaster ride of building a business, I discovered that resilience is born from this partnership. By harnessing the power of both, I transformed my pain into purpose, allowing me to overcome the challenges of AFib and entrepreneurship, and ultimately, to help others do the same.”
Executives on the Move
Frederick Beddingfield III, M.D., Ph.D. Joins Neurodon Board of Directors
Dr. Beddingfield is a 22-year veteran of the biopharma industry

SynerFuse® Announces Chief Technology Officer | Michael Park, M.D., Ph.D.
Dr. Park is a former principal investigator for the SynerFuse® proof-of-concept study and primary inventor of SynerFuse® technology.
Clinilabs Expands Executive Leadership Team With Key Appointments
Clinilabs, LLC, a leading full-service contract research organization (CRO) specializing in central nervous system (CNS) drug and medical technology development, today announced the appointments of
FDA Medical Device Updates
Aidoc Secures Landmark FDA Clearance for 1st Foundation Model-Powered Clinical AI Solution of Its Kind
“This isn’t just an upgrade, it’s a paradigm shift,” said Reut Yalon, PhD, Chief Product Officer, Aidoc. “Our goal is twofold: continuously enhance existing solutions with this new technology while simultaneously pushing AI into new frontiers and expanding coverage across a broader range of conditions. This clearance is just the beginning.”
U.S. FDA Grants Orphan Drug Designation to Ariceum Therapeutics’ Proprietary Radiopharmaceutical Cancer Therapy
Manfred Rüdiger, Chief Executive Officer at Ariceum Therapeutics, said: “Receiving ODD for 225Ac-satoreotide is a recognition of its potential as a treatment option for patients with SCLC and an important regulatory milestone for Ariceum. The FDA’s ODD will support our objective to accelerate the development of 225Ac-satoreotide through human trials to provide a potentially life-saving therapy to patients with limited alternatives.”
Huxley Medical’s SANSA Home Sleep Apnea Test Gets FDA Nod
In a clinical trial involving 340 patients across seven institutions, SANSA obtained its initial FDA clearance in 2024, demonstrating its accuracy in comparison to the gold-standard polysomnography. This innovative test incorporates nine distinct physiological channels, such as oximetry, respiratory effort, sleep/wake staging, and a reference electrocardiogram (ECG), into a single patch worn on the chest. This integration allows for a comprehensive understanding of cardiopulmonary health and provides valuable insights.
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
Boston Scientific Reveals Agreement to Acquire SoniVie Ltd.
“Renal denervation for hypertension is an exciting medical advancement for the millions of patients it may help and is supported by positive results from contemporary clinical trials and ongoing research,” said Lance Bates, senior vice president and president, Interventional Cardiology Therapies, Boston Scientific. “We believe the addition of the differentiated, ultrasound-based TIVUS system can complement our expansive interventional portfolio with a minimally invasive therapy for patients with hypertension and provides opportunity for future advancements in this space.”
OrganOx Completes $142 Million Equity Financing Led by HealthQuest Capital
The oversubscribed round was led by new investor HealthQuest Capital with support from existing OrganOx investors BGF and Lauxera Capital Partners, along with additional new investors Sofina, Soleus Capital, and Avidity Partners.
Advancing Eye Health: Perceive Pharma Secures $15M Investment Boost
In addition, Perceive Pharma announced that Carol Gallagher, Pharm.D. has joined the company’s Board of Directors as an independent director.
Exero Medical Closes on $12.7M Preferred Seed Financing
This financing includes investments from new investors Longevity Venture Partners, Tech Council Ventures, and Edge Medical Ventures, alongside participation from existing investors notes Exero Medical.
Market Reports
Advancements In Imaging
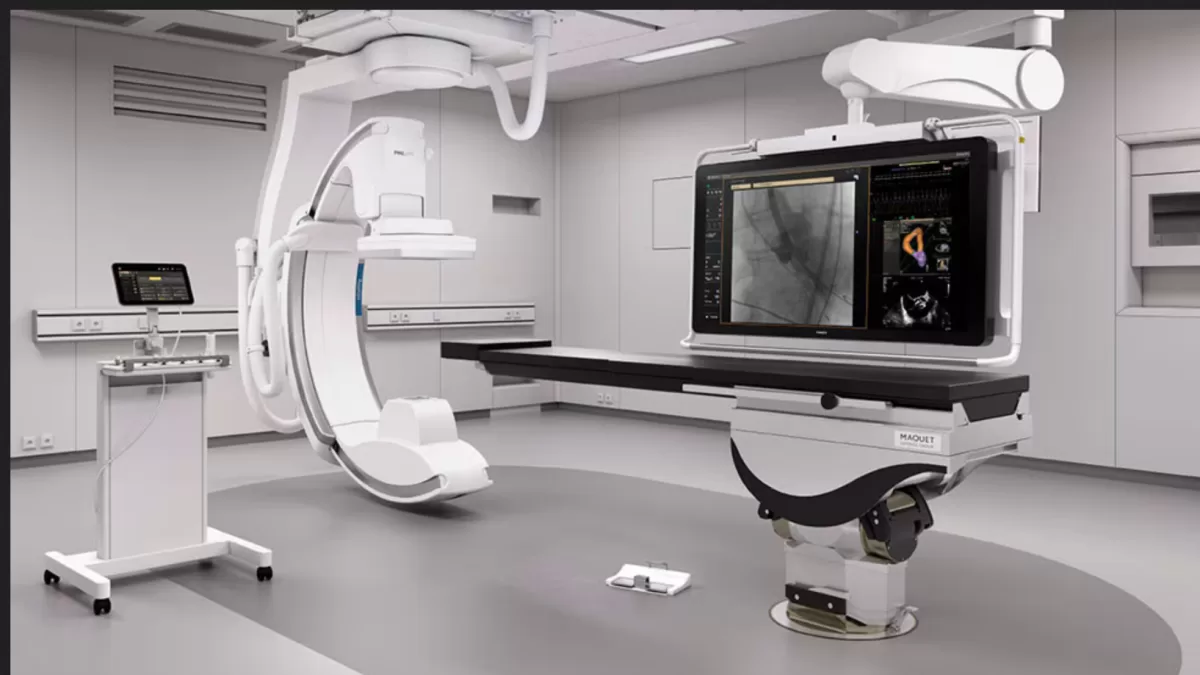
Carilion Clinic Further Expands Quality Cardiac Care Access with Latest Philips Innovations at New Regional Cardiovascular Institute in Virginia, US
“At Carilion Clinic, we are committed to improving the health of the communities we serve. We know that collaboration is an essential component to realizing this vision,” said Marguerite Underwood, Vice President for the Carilion Clinic Cardiovascular Institute. “Working with Philips ensures that we will be able to set the standard in cardiovascular care, not only today, but well into the future.”
Bon Secours Mercy Health and Philips Sign Multi-Year Strategic Collaboration
“This collaboration is part of our commitment to drive improved healthcare quality while reducing costs and addressing healthcare issues facing entire communities,” said Jodi Pahl, Chief Nursing Officer for workforce experience and nursing outcomes, Bon Secours Mercy Health. “This 10-year journey will bring innovations that will transform care delivery.”
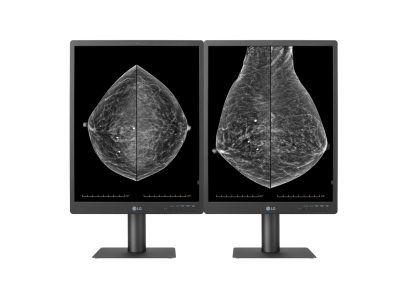
LG Electronics Launches 5MP Diagnostic Monitor for Breast Imaging
LG Electronics reports: Intended for digital mammography and digital breast tomosynthesis, the 21HQ613D-B is a 21.3-inch 5-megapixel (MP) IPS diagnostic monitor designed to deliver high-definition radiological images and maintains consistent image quality through its internal front calibration sensor and calibration software.
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

The EHR + RCM Combo That Mental Health Practices Have Been Waiting For
Integrating EHR and RCM systems created for mental health isn’t just a step up on technology—it is also rethinking care and administration coming together.

How ABA Billing Services Drive a Healthier Revenue Cycle for Therapy Practices
In ABA therapy, delivering quality care is the goal—but sustaining that care depends on something just as critical: a steady, healthy revenue cycle.

Building Better Care: How Healthcare Facilities Can Support Disability Needs
As the healthcare landscape shifts to better meet the needs of a diverse population, qualified providers are essential to ensuring that these needs are met.

Leveraging Technology To Streamline Patient Billing Processes
Healthcare organizations are increasingly turning to innovative technology solutions to revolutionize their patient billing processes and optimize revenue cycle management. Modern healthcare billing faces unprecedented
Healthcare Careers
Career Opportunities for Dental Professionals: 7 Pathways
There are numerous career opportunities for dental professionals out there. Typically, there are three directions people in this medical field can focus their efforts on:
Exploring Dental Hygiene As A Career
Dental hygienists are oral healthcare professionals responsible for cleaning teeth, educating patients on proper oral hygiene, and assisting dentists during various procedures. You might consider

A Small Guide On How To Become A Medical Assistant
When most people think of healthcare workers, their minds go to doctors, nurses, and surgeons. However, there are many other positions and jobs that are
Nurses Corner
AIS Healthcare Celebrates National Nurses Month
AIS Healthcare nurses hold certifications in specialty infusion care and undergo advanced and ongoing clinical training, allowing them to provide patients with the best possible care.

Collette Health Appoints Christine Gall as Chief Nursing Officer
Christine Gall will oversee all clinical operations and innovation initiatives to further enhance Collette Health’s commitment to helping hospitals integrate virtual patient safety solutions to prevent falls and adverse events and improve patient outcomes.

Symtech Systems Announces Nurse Call Systems Expected to Surge in Market Growth in 2024
By partnering with Symtech Solutions, healthcare facilities can benefit from cutting-edge nurse call systems that enhance patient care and increase staff productivity. The company’s commitment to delivering the best solutions combined with its industry expertise positions them as a trusted and reliable provider in the market