- 1st Surgery With Polymer-Based Coil Takes Place, Using Embolization, Inc., Device
- Brain Balance in Wayne Pennsylvania
- Press Ganey report reveals the trust factors reshaping how consumers make healthcare decisions
- Amae Health Announces $25 Million Series B to Advance Toward a Cure for Severe Mental Illness
- Locumsmart Names Thomas Lanvers President
- Channel Medsystems Surpasses 5,000 Cerene® Cryotherapy Procedures, Marking Milestone in Women’s Health Innovation
- BUILD aims to advance the implementation of person-centred integrated long-term care across Europe
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
Spinal Elements Awarded Vizient Innovative Technology Contract for Karma® Fixation System
The contract was awarded based on the recommendation of the Karma Fixation System by hospital experts who serve on one of Vizient’s client-led councils, and it signifies to Vizient provider clients unique qualities that potentially bring improvement to the healthcare industry
Ellipsis Health Selected as Key Partner in CMS Health Tech Ecosystem to Drive National Healthcare AI Transformation
The company, represented by CEO Mainul Mondal, joined CMS Administrator Dr. Mehmet Oz, federal leadership, and a select group of leading health systems, payors, and innovators, in Washington, D.C. to tackle developments in healthcare that serve the benefit of all patients.
Norwalt Automation Group Partners with ei3 to Launch Nexus: A Smarter, Data-Driven Future for Manufacturing Automation
Manufacturers can now achieve 24/7 operational intelligence and predictive maintenance without additional IT staff through this AI-powered IIoT integration
Hohenstein Medical Earns GLP Certification for Medical Device Testing
Good Laboratory Practice certification expands company’s ability to support medical device manufacturers in meeting the complex demands of international regulatory frameworks.
Clinical Trials
Second Phase of ADVANTAGE AF study of FARAPULSE ™ Pulsed Field Ablation System Meets Primary Safety and Efficacy Endpoints
Trial achieves positive results in the treatment of persistent atrial fibrillation.
CorWave Completes 6-Month In Vivo Study for Clinical Trials Initiation
In vivo studies demonstrated the pump’s successful operation for a period of up to six months. Additionally, nine chronic ovine implants were conducted for 60 days with no device failure or sign of thrombosis at explant. These significant milestones mark the final stage of CorWave’s preclinical development, paving the way for its First-In-Human study.
Vektor Medical Announces Post-Market Study Evaluating Benefit of AI-Powered Analysis to Identify and Ablate Non-Pulmonary Vein AF Targets
vMap is the only FDA-cleared non-invasive AI-powered arrhythmia analysis technology designed to improve, enhance, and accelerate ablation procedures, utilizing data from a standard 12-lead ECG.
Biotechnology News
Nuclera Establishes a Distribution Network Across APAC and the Middle East
Operational expansion accelerates global distribution capability to increase regional access and technical support. Multiple partnerships establish direct distribution channels in China, Japan, South Korea, Singapore and Israel.

Seegene’s CURECA(TM) Emerges as a Potential Game Changer in End-to-End PCR Automation
With a fully modular design, CURECA introduces a bold vision for automating pre-treatment across diverse PCR specimen types
VERAXA Biotech Enters Co-discovery Alliance with OmniAb for a Novel Bispecific Antibody Drug Conjugate Program
Partnership Combines VERAXA’s Proprietary ADC Technology with OmniAb’s Antibody Discovery Technology for Novel Therapies Targeting Solid Tumors.
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!
Focusing Solely on US Patents Can Be a Risky Move. Here’s Why | By Christopher Cauble
Christopher Cauble’s view is that the U.S. healthcare market is big enough that good profits can be made without having to bother with the hassle of foreign patent protection, particularly patent protection in China. Read on to learn more.

The Perils of Over-Regulating AI in Healthcare and Life Sciences | By Adnan Masood, PhD. Chief AI Architect
As an AI researcher and practitioner deeply embedded in the healthcare and life sciences sectors, Dr. Masood sees the passage of California’s SB 1047 as a complex issue with significant implications. He writes this controversial bill, now heading to Governor Newsom’s desk, represents a fundamental misunderstanding of AI as a general-purpose technology. It attempts to regulate the technology itself rather than focusing on specific, problematic applications.

Advancing Fully Implanted Hearing Systems | By Brent Lucas, CEO of Envoy Medical
Envoy Medical has taken a different approach with its fully implanted hearing devices, and it looks to turn the industry on its… ear. “Listen” to what Brent Lucas, CEO of the company has to say.
Executives on the Move

Rad AI Names Demetri Giannikopoulos as Chief Growth Officer to Accelerate AI-Driven Radiology Healthcare Growth
Mr. Demetri brings over 20 years of experience in healthcare technology, with a track record of advancing AI adoption in complex clinical settings.
RapidRatings Appoints Charlie Minutella as CEO to Accelerate Strategic Growth and Innovation
Charlie Minutella brings a wealth of industry knowledge and a strong track record of leadership, innovation, and operational excellence. His appointment ushers in a new chapter for RapidRatings as it continues to shape the future of financial risk intelligence.

Dr Ray Kotwicki, Chief Medical Officer of Hightop Health, Appointed President of the Southern Psychiatric Association
Dr Ray Kotwicki will lead the SPA into its next chapter, focusing on education, collaboration, and advocacy for psychiatrists across the region.
FDA Medical Device Updates
RapidAI Secures FDA 510(k) Clearance for Groundbreaking AI 3D Head and Neck CTA Imaging: Lumina 3D™
RapidAI continues to pioneer AI advances by revolutionizing 3D imaging with no-click, AI-powered reconstruction.
Caranx Medical Announces FDA Submission of TAVIPILOT Soft: the World’s first AI Software for Real-time Intra-operative Guidance of Transcatheter Heart Valve Implantation
“This first release of our TAVIPILOT software marks an important step toward AI-augmented procedures. Our software is designed to be user-friendly, ensuring that cardiologists and surgeons can integrate it seamlessly into their usual practice. TAVIPILOT Soft allows clinicians to position a prosthetic valve easily and with high precision, ensuring that more patients could benefit from a high quality of care.” says Caranx Medical co-founder and CTO Pierre Berthet-Rayne.
Vergent Bioscience Receives FDA Fast Track Designation for Abenacianine for Injection (VGT-309) to Help Surgeons Visualize Tumors in the Lung During Surgery
“Receiving Fast Track designation from the FDA reinforces the potential of abenacianine to address existing deficits in lung cancer surgery by helping surgeons better visualize tumors in the lung during minimally invasive surgical procedures,” said John Santini, Ph.D., president and chief executive officer at Vergent Bioscience. “We look forward to collaborating with the FDA to make abenacianine available to surgeons and their patients as quickly as possible.”
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
REEV Raises $9.2M | To Revolutionize Mobility Assistance for Patients with Gait Disorders
“With the support of our world-class partners, we are thrilled to enter the next phase of clinical and industrial development,” said Amaury Ciurana, Co-Founder and CEO of REEV. “Our innovative technologies, like the DREEVEN motorized orthosis, aim to redefine mobility assistance, transforming lives and reshaping the orthotics industry.”
Glox Therapeutics Awarded Share of £3M Collaborative Discovery Programme Funding from Cystic Fibrosis Antimicrobial Resistance Syndicate
Glox Therapeutics will receive up to £500,000 of the fund to accelerate the development of its novel precision antibiotics, helping to address the growing need for effective treatments to overcome antimicrobial-resistant lung infections in people with cystic fibrosis.
NAMSA Acquires the U.S. Medical Device Testing Operations of WuXi AppTec, Solidifying its Position as the Leader in MedTech Testing and Clinical Research
Under the agreement, NAMSA will acquire WuXi AppTec facilities in Minnesota and Georgia.
Caidya Announces $165 Million Strategic Growth Investment from Rubicon Founders
“We look forward to partnering with Caidya’s management team and existing investors to support the company’s continued growth plans as a leading global CRO,” said Glaccum. “As both investors and operators in the global healthcare and pharmaceutical services industries, we are excited to bring our expertise to support Caidya’s mission to advance the future of healthcare by providing patients around the world with access to novel therapies.”
Market Reports
Advancements In Imaging
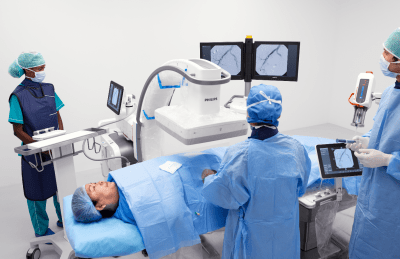
Philips Zenition 90 Motorized receives FDA 510(k) Clearance
“During complex procedures, it’s vital to be able to rely on surgical imaging systems. As clinicians navigate their way through challenging anatomy, the priority is to quickly visualize small anatomical details while limiting X-ray dose,” said Mark Stoffels, Business Leader Philips Image Guided Therapy Systems. “The new Zenition 90 Motorized empowers medical teams to confidently perform a wide range of interventions while achieving the best possible outcome for their patients.”
VUZE Medical Announces U.S. FDA 510(K) Clearance for Second-Generation Software-Based 3D Guidance System for Spine Surgery
VUZE 2.0 provides much-extended interoperability and functionality; like VUZE 1.0, requires only a 2D C-arm and PC in the OR — no cameras, markers, references or tool add-ons.

Nicklaus Children’s Health System, Philips Will Provide Innovative Patient & Staff Experience, Helping to Enhance Outcomes for the Smallest of Patients
Philips AI-enabled diagnostic and experiential technologies help put patients at ease while empowering staff with faster, high-quality scans and increased diagnostic confidence.
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

Navigating the Digital World: What Doctors Are Doing About Bad Reviews Online
Positive and negative online reviews can be managed in various ways. In this article, we’ll explore how doctors manage their online reputation and learn how to respond to negative patient reviews.

The Benefits of Using EMR for Mental Health Practices
EMR systems enable mental health professionals to maintain and access patient records with ease, creating a more efficient workflow and better patient outcomes.

The Future of Cybersecurity: How AI and Machine Learning Are Changing the Game
As cyber threats become more sophisticated, organizations must adapt by leveraging advanced technologies to protect their data and systems. AI and ML are transforming the way cybersecurity professionals detect, respond to, and prevent cyberattacks, providing more efficient and proactive security solutions.

Protecting Surgeons’ Vision: The Critical Role of Laser Safety Glasses in Modern Operating Rooms
Surgeons face numerous risks during procedures, with eye protection being a paramount concern. Protecting surgeons’ vision is crucial, and laser safety glasses play a critical
Healthcare Careers

Five Ways Health Professionals Can Benefit From Field Research
Field research is often associated with academic research and studies, but it can also be an essential job asset for health professionals. Field research involves

The Path to a Medical Career: A Comprehensive Guide to Studying Medicine
Studying medicine is no easy feat. It requires hard work, dedication, and a passion for helping others. But if you’re willing to put in the
Cardiologists: Who They Are & What They Do
There is a fine line between getting sick and suffering from illness. Your physician can help you get better with medicines when you are sick
Nurses Corner

How Postgraduate Nursing Education Improves Patient Outcomes
In this article, we’ll examine the advantages of postgraduate education for nurses so that you can decide whether it’s the right path to take for your career.
University of Phoenix College of Nursing Leadership Selected for Prestigious Podium Presentation at AACN’s 2024 Diversity Symposium
University of Phoenix College of Nursing incorporates diversity, equity, inclusion and belonging (DEIB) into its core values and curriculum, aligning with the broader university initiatives. The selected abstract delves into the challenges and triumphs encountered on the journey to inclusivity in nursing education, shedding light on the dynamic intersection of policy evolution and fostering diversity within the nursing profession.
Navigating Vet Nurse Salaries in Australia: Trends and Predictions
The vet nurse salaries in Australia can vary based on several factors, including geographical location, experience, and the specific demands of the job. Staying abreast of these trends helps veterinary nurses in Australia make informed decisions about their career paths and potential earnings.


![Nooro Foot Massager Reviews 2024– Scam Or Legit, Instructions, & “Consumer Reports 100%” [WARNING!] By Reddit? - Medical Device News Magazine Nooro Foot Massager](https://infomeddnews.com/wp-content/uploads/2024/01/nooro-foot-400x271.png)
