- Wysa Partners with RxCap to Advance Medication Adherence
- ACIP Recommends Use of Merck’s ENFLONSIA for Prevention of Respiratory Syncytial Virus Lower Respiratory Tract Disease in Infants Younger than 8 Months of Age Born During or Entering Their First RSV Season
- How Fiber Laser Cutting Machines Are Used in Medical Equipment Manufacturing
- Liviniti Achieves HITRUST i1 Certification for Industry-leading Security Practices
- STAAR Surgical Announces Appointment of Deborah Andrews as CFO | Forms Capital Stewardship Committee of the Board of Directors
- Breakthrough in Patient-Derived Stem Cells for Therapeutic Regenerative Medicine
- Acera Surgical Broadens Reach of Restrata ® with New FDA Clearance for Soft Tissue Reinforcement
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
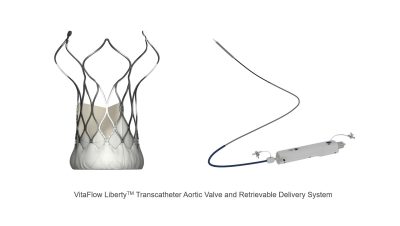
VitaFlow Liberty ™ Granted EU CE-MDR Mark
Jeff Lindstrom, President of CardioFlow, stated, “The certification of VitaFlow Liberty by the CE regulatory body under MDR, is a testament to CardioFlow’s world-class R&D, quality, and clinical capabilities. This recognition will expedite the global clinical adoption of the VitaFlowTM series along with other innovative products, advancing CardioFlow’s globalization strategy. This achievement also positions us to make a more substantial contributions to developments in the field of heart valve interventions, ultimately benefiting patients across the globe.”
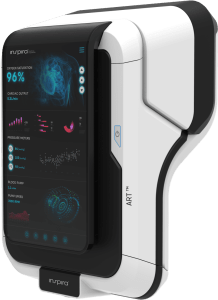
Inspira Receives 1st Ever Purchase Order for INSPIRA™ ART100 Systems in the U.S.
Inspira reports the first units are expected to be shipped in Q4 2024. The Purchase Order follows the recent FDA Clearance of the INSPIRA™ ART100.
Sharps Technology Receives $30 Million Purchase Order for Prefillable Copolymer Syringes to be Manufactured at SC Facility
The purchase order includes deliveries for both the 10mL and 50mL specialty copolymer prefillable syringes that will be manufactured at the West Columbia site being acquired from Nephron. This assurance of supply is expected to support the planned expansion of the 503b operations at the Nephron site. The new copolymer syringe technology reflects the pharmaceutical and healthcare industry’s trend toward transitioning injectable drug therapies into innovative polymer prefillable syringes and away from the older glass and standard plastic syringes reports Sharps Technology.
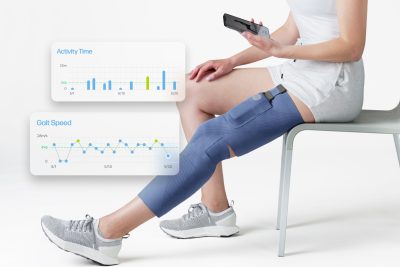
CIONIC Unveils Three New Centers of Excellence on the East Coast to Aid Patient Mobility Through the Use of FDA-Cleared Bionic Clothing and Neuromodulation
Jeremiah Robison, CIONIC Founder & CEO. “We are proud to work with IMSMP, Jersey Shore University Medical Center, and MedStar Health to deliver a unified care experience with the Neural Sleeve from the clinic to the home, setting a new standard for mobility care.”
Clinical Trials
BENEFIT-01 Study Now Published in Neuromodulation: A Glimpse into the Future of Spinal Cord Stimulation Programming
“BENEFIT-01 Study was the first in a series of studies that speaks to our commitment to advancing and sharing the science behind SCS. It’s exciting to see these learnings realized as patients experience the positive impact of RESONANCE™ stimulation with our Prospera™ SCS system,” said Todd Langevin.
Sensorion Announces Approval to Initiate Lead Gene Therapy Candidate SENS-501 (OTOF-GT) into a Phase 1/2 Clinical Trial in some European Countries
Sensorion notes This gene therapy for patients suffering from otoferlin deficiency has been developed in the framework of RHU AUDINNOVE, a consortium composed of Sensorion with the Necker Enfants Malades Hospital, the Institut Pasteur, and the Fondation pour l’Audition. The project is partially financed by the French National Research Agency, through the “investing for the future” program (ref: ANR-18-RHUS-0007).
Orlucent Demonstrates Accurate, Non-Invasive Detection in vivo of Melanoma-Related Activity Using First-in-Kind Handheld Mole Imaging System
The Orlucent system will be the first to provide clinicians with a noninvasive direct measure of early intra-cellular activity and shows promise to transform the landscape of identifying melanoma at its earliest stage.
Biotechnology News
Hyundai Bioscience Announces Clinical Development Plan for Niclosamide-based Metabolic Anticancer Drug Targeting P53 Mutation Cancer
Sang-ki Oh, CEO of Hyundai Bioscience, stated, “Niclosamide-based metabolic anticancer drug candidate will be the first P53-targeting anticancer treatment that selectively kills p53 mutated cancer cells,” and added, “Through our subsidiary ADM Korea, we plan to conduct clinical trials targeting cancer patients with intractable cancer caused by p53 mutations, which will be the first step of clinical development on niclosamide-based anticancer agent pipeline.”
Preci and Biopredic International Partner for Higher Performance of Suspended Pooled Hepatocytes with Extended Longevity and Large-scale Availability
Under a license agreement, Biopredic will leverage Preci’s expertise and production capacity in sourcing primary hepatocytes, and combine with its own IP and know-how in cell pooling. The partnership will provide DMPK researchers access to large batches of high-performing suspended pooled hepatocytes with extended longevity from multiple donors.
The Future of Biotechnology: Insights Into Cutting-Edge Developments
The future of biotechnology holds immense promise, with continued advancements driving innovation and progress across various sectors. From gene editing and synthetic biology to biopharmaceuticals and beyond, the possibilities are endless. By staying informed, collaborating with industry leaders, and leveraging cutting-edge technologies, we can harness the power of biotechnology to address some of the most pressing challenges facing humanity.
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!
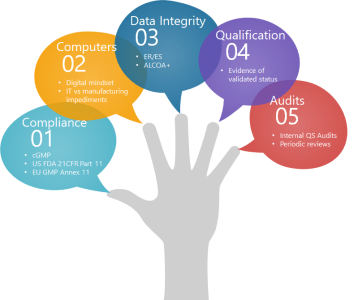
A Volte-face on Introgressed and Adaptive Validation: CSV Implementation In MedTech Industry
The use of computer systems has been considered a strategically distinctive option for the entire transition to digital data management. The gap between the paper-based and the electronic records calls into question how the MedTech industry can ensure the compliant data management. The computer systems enable granular visibility of the enterprise operations and insights beyond the production floor and efficiently adapts to computing requirement peaks and valleys.

Abdominal Aortic Aneurysm Treatment Needs to Advance Using AI By Dr Patrick Muck, Vascular Surgeon, TriHealth
Dr Patrick Muck writes ” Several players are working to incorporate AI into their care modules. At Viz.ai, where I serve as an advisor, they recently received the first FDA clearance for an AI-powered solution for the detection and triage of suspected AAA. This algorithm leverages AI to automatically search for the presence of a suspected abdominal aortic aneurysm (AAA) from any computed tomography angiography (CTA) from any scanner in any institution in a hospital network that uses the company’s platform.

Mother Nurture™ Custom Flexible Orthotics Top the List of Baby Shower Registries: By Jamie Greenawalt, President, Foot Levelers
Custom, flexible orthotics rely upon natural pain relief, allowing women to forego pharmaceutical intervention – an important concern since the physiology of pregnancy affects the pharmacokinetics of medications used, and certain medications can reach the fetus and cause harm.
Executives on the Move
FDA Medical Device Updates
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
Ceros Financial Services Arranges $7.5 Million Financing for Wesper
Wesper Home Sleep Lab features a wearable device that monitors sleep conditions For clinical-grade evaluation, analysis and treatment.
NICO Raises $12.5 Million in Oversubscribed Investment Round
NICO is an Indianapolis-based medical device innovator creating a new reality in neurosurgery through its MIS technologies. The company has a strong shareholder base that has invested more than $62 million since its inception in 2007.
Cresilon Closes $25 Million in Series A-4 Financing to Accelerate Global Expansion and Bring Revolutionary Hemostatic Gel to Human Health Market
Cresilon advises the financing will support the company’s efforts to aggressively accelerate its global expansion plan and bring its revolutionary hemostatic gel to the human health market.
Monteris Medical Announces $73 Million in Combined Equity and Debt Financing
Monteris Medical notes the proceeds of this financing will be used to continue to support the rapid adoption of the NeuroBlate® System, an MR-guided laser ablation system that allows for a minimally invasive surgical alternative to open craniotomies.
Market Reports
Advancements In Imaging
Qmetrics Technologies and 20/15 Visioneers Announce Go-to-Market Partnership
“Concussions are largely diagnosed and managed via patient symptoms,” says Edward Schreyer, CEO, Qmetrics Technologies. “Post-concussive brain injuries are typically too subtle to be seen by the human eye. Our technology-driven Concussion Index uses algorithms to identify post-concussive injuries.

KA Imaging Unveils New Brand Identify for its Patented Dual Energy Technology
KA Imaging’s SpectralDR™ technology enables dual-energy subtraction, providing bone and tissue differentiation with a single standard X-ray exposure.
Carestream Shares Path to Accelerated Delivery of AI Features at HPE Discover 2022 Conference
Carestream leverages HPE GreenLake’s enterprise-grade cloud service for machine learning (ML Ops platform) to accelerate development of its AI applications for medical imaging.
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

How to Enhance Patient Trust in Your Healthcare Business
We all understand that trusting our healthcare providers is of the utmost importance when it comes to our well-being. It forms the foundation of a

Material Matters: Understanding The Best Fabrics For Medical Scrubs
In healthcare, medical professionals rely on various essential tools and equipment to perform their duties effectively. Among these, the humble yet indispensable medical scrubs play

Building A Successful Dental Website: The Essential Role Of SEO
There is no denying that every business needs a well-designed, easy-to-use website—dental practices included. More than just a digital billboard, your website can serve as
![image[2] - Medical Device News Magazine Software Development Life Cycle](https://infomeddnews.com/wp-content/uploads/2023/06/image2-400x283.jpg)
Navigate the Software Development Life Cycle with Expertise
In the realm of software development, the Software Development Life Cycle (SDLC) serves as a guiding framework for creating high-quality software solutions. It encompasses a