- CPR Therapeutics Receives Follow-on U.S. Patent for Integration of Defibrillation into an Automated CPR Device
- Piramal Pharma Ltd. Continues to Invest In USA; $90M in Expansions of Two US Sites
- Thirona and COPD Foundation Partner to Advance AI-Driven Phenotyping of COPD Patients
- Trogenix Further Strengthens its Scientific Advisory Board with the Appointment of World-Leading Experts, Prof Nada Jabado and Prof Burkhard Becher
- Guided Spinal Navigation Landmark Surgery Opens the Door to Cost-Effective 3D Augmented Reality
- Nihon Kohden Payment Solutions Launches to Help Healthcare Facilities Acquire Critical Tech
- United Imaging – Innovations That Are Changing the World of Medical Diagnostics
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
Aspen Laser Named as Space Certification Program Partner by the Space Foundation
The Space Certification Program awards a “seal of approval” to commercial companies that demonstrate products and services in technology, data, education and creative markets that originate from space technology and are a source of inspiration for discoveries and innovations in the global space ecosystem.
WIRION Embolic Protection System – 1st Patients Treated Reports Cardiovascular Systems, Inc.
WIRION Embolic Protection System is a distal embolic protection filter used to capture thrombus and debris that can be associated with all types of peripheral vascular intervention procedures, including atherectomy.
Zenith Award from American Association for Respiratory Care for Support of Respiratory Therapists Is Awarded to Vapotherm
Award celebrates excellence in support and quality as chosen by AARC member votes
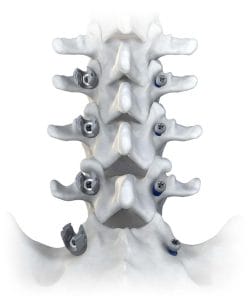
Accelus Introduces LineSider Modular-Cortical System for Posterior Fixation
Accelus notes designed for greater procedural visibility, versatility and efficiency, the modular screw design provides surgeons with enhanced visualization by allowing the placement of screw shanks early in the procedure, followed by modular tulips and rods at the end.
Clinical Trials
Oncolytics Biotech Presents Positive Updated Pancreatic Cancer Data from GOBLET Phase 1/2 Study at ESMO
“The data from this study show a correlation between the expansion of TIL-specific clones and tumor response, which provides compelling support for the use of pelareorep-based therapies in immunologically “cold” tumors. In particular, these data, along with the impressive clinical results, support the ability of pelareorep-checkpoint inhibitor combination therapies to meaningfully improve treatment responses in diseases like pancreatic cancer that have resisted immune-based therapeutic approaches,” said Thomas Heineman, M.D., Ph.D., Chief Medical Officer at Oncolytics Biotech.
New Phase 3 TREMFYA® (guselkumab) Results in Ulcerative Colitis Show a 77 Percent Overall Clinical Response Rate and Early Symptom Improvement
“Ulcerative colitis is a complex immune-mediated disease that can cause a wide range of often-debilitating symptoms,” said study author Jessica R. Allegretti, M.D., Medical Director, Crohn’s and Colitis Center at the Brigham and Women’s Hospital, Boston, MA, USA.c “Results from the QUASAR studies support the potential of TREMFYA as a durable and fast-acting treatment option.”
Aculys Pharma Delivers Positive Phase 3 Clinical Study Interim Analysis result of a Diazepam Nasal Spray | An Antiepileptic Drug for RX of Epileptic Seizures
Diazepam nasal spray is Japan’s first nasally administered antiepileptic that has demonstrated efficacy and safety in Phase 3 clinical trials in Japan for the treatment of status epilepticus or epileptic seizures that may lead to status epilepticus.
Biotechnology News
From Lab to Clinic: Streamlining Vaccine Development and Production
The acceleration of vaccine development and the optimization of production methods are essential in addressing global health challenges and ensuring timely access to life-saving vaccines
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

To Prepare for Future Surges, U.S. Hospitals Must Start Planning — & Sharing Resources—Now! By Regina E. Herzlinger
In the age of pandemics, how can we fix our hospital capacity problem? Ms. Herzlinger dives right in with solutions.

New Biosensing Technology Provides a Solution for Dehydration in Older Adults: By Jennifer Corso, Upstream Marketing Manager, Physiology, Rockley Photonics
Age-related physiological changes can impact a person’s ability to remain hydrated, such as a decline in kidney function and worse. A biomarker-sensing platform makes it possible to measure hydration noninvasively and constantly. It can recommend real-time steps for managing hydration in older people according to their particular needs. Read on.

Reducing Hospital Acquired Infections In A Post-Pandemic World By: Jason DiBona, CEO of AeroClean
Jason DiBona provides in-depth details on reducing hospital-acquired infections with Improved Indoor Air Quality (IAQ). What hospitals and healthcare institutions need to know.
Executives on the Move
FDA Medical Device Updates
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
Aspen Surgical Products Receives Additional Investment from Audax Private Equity
Aspen Surgical Products CEO Jason Krieser said, “This transaction represents an excellent return for our original investors, while empowering our Aspen Surgical organization to continue to progress in our mission of improving safety and efficiency for clinicians at an even faster rate.”

CardioRenal Raises €3.3 Mil
CardioRenal advises the funds will enable the company to complete the process of obtaining regulatory approval for its connected medical device.
Osso VR Raises $66 Million
Ossor VR will use the funds to broaden access to surgical education for healthcare professionals.
Podimetrics Secures $45 Million Series C
Podimetrics said existing investors include, Polaris Partners and Scientific Health Development, also participated in the financing.
Market Reports
Advancements In Imaging
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications
How to Migrate to a Cloud-Based Laboratory Information System
Migrating to a cloud-based laboratory information system (LIS) can be a daunting task for laboratory managers, IT administrators, and healthcare professionals. With the right knowledge

Eight Reasons Continuing Medical Education is Important
Medical knowledge constantly evolves, and healthcare professionals must stay up-to-date with the latest advancements and techniques. It is where continuing medical education (CME) comes in,

How To Find The Right Marketing Agency For Your Healthcare Business
In today’s competitive healthcare landscape, finding the right marketing agency to help your business stand out is crucial. This blog aims to help you streamline

What Spa Owners Should Look for When Buying A Cryo Body Sculpting Machine
Cryo body sculpting equipment has become highly trendy lately due to its non-invasive and successful body sculpting procedures. As a spa owner, purchasing a cryo