- Monteris Medical and Symphony Robotics Announce Strategic Collaboration to Launch AI-Driven Micro-Robotic Platform for Laser Ablation in the Brain
- Asahi Kasei Elevates Critical Care Solutions with FDA Approval of ZOLL’s Zenix Monitor/Defibrillator
- Field Medical | First-in-Human 6-Month Data Selected for Late-Breaking Clinical Trial at VT Symposium 2025
- Summit Cancer Center Director, Arvind Chaudhry, MD, PhD, to Chair Coeur d’Alene Hematology Oncology Conference Focused on Precision-Guided Cancer Care
- Italfarmaco Appoints Francesco Di Marco as CEO
- Santhera Secures Agreement with Biomedica for the Distribution of AGAMREE® (Vamorolone) in Russia
- Zelluna Receives Positive MHRA Feedback and Strengthens UK Clinical Strategy for ZI-MA4-1 Paving the Way for First-in-Human Trials in the UK
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
Ethris Signs Strategic Collaboration with Thermo Fisher Scientific to Provide Access to mRNA Technology Platforms
Partnership further validates Ethris’ novel technology platform based on stabilized non-immunogenic mRNA, which overcomes the innate instability and immunity of mRNA.
InspireMD Announces CE Mark Approval for CGuard ® Prime Embolic Prevention System (EPS) Under European MDR for the Prevention of Stroke
CGuard® Prime was developed incorporating extensive user feedback and optimizes deliverability and deployment of the proven CGuard stent.
Nectero Medical Secures Category III CPT Codes for Investigational Therapy to Treat Small- to Medium-Sized AAAs
This designation represents a key milestone toward future reimbursement and broader clinical adoption.
Medtronic Announces MiniMed as Name for Planned New Diabetes Company
The name honors the company’s roots, reflecting its original name prior to its acquisition by Medtronic in 2001, and a deep 40-year history of being at the forefront of transforming diabetes care around the world.
Clinical Trials
50% of Older Adults to Rely on Wearables for Life-Saving Alerts by 2034, ScienceSoft Predicts
The focal point of the study is the growing role of machine learning in wearables. Peer-reviewed research from PubMed, MDPI, ScienceDirect, and other leading sources for scientific and medical research proves that monitoring vital signs, such as heart rate, can support the early detection of health risks and potentially reduce reliance on traditional in-person medical examinations.
SamanTree Medical Highlights Research Showing 67% Reduction in Reoperation Rates for Breast-Conserving Surgery at the 2024 SABCS
“These outstanding results underscore the potential of the Histolog Scanner to enhance oncologic surgical outcomes significantly,” said Olivier Delporte, CEO of SamanTree Medical. “By enabling real-time, high-resolution tissue microstructure assessments during BCS, our technology addresses a critical unmet need, reducing reoperation rates and improving patient outcomes.
New Data From SonarMD Finds that Anxiety Increases Risks for Inflammatory Bowel Disease Flares and Disease Severity
“These findings demonstrate that mental health factors like anxiety play a critical role in the clinical course of IBD,” said Beth Houck, CEO, SonarMD “By identifying and addressing psychological contributors, we can refine value-based care models to better support patients with chronic digestive diseases. Integrated care models that incorporate mental health screening and interventions should be considered a standard for high-quality IBD care.”
Biotechnology News
COEPTIS’ NexGenAI Affiliates Partners with NUBURU Network to Drive Innovation in AI and Robotics as Part of its Transformation Plan
Dave Mehalick, CEO of COEPTIS stated, “Partnering with NUBURU marks a significant step in COEPTIS’ journey toward pioneering innovative technology solutions. By harnessing the power of NexGen’s AI-driven capabilities, we are poised to not only enhance our own operational efficiencies but also redefine how businesses engage with their clients in the rapidly evolving defense and security landscape. This collaboration underscores our commitment to fostering growth through advanced technological integrations.”
AION Labs, In Continued Partnership with BioMed X, Launches 2025 Global Call for Applications: Generative AI for Novel Target Combinations
Title
AION Labs Announces 2025 Global Call for AI Applications
Description
AION Labs partners with BioMed X to launch an AI-driven platform for innovative medical device applications in 2025.
Wren Laboratories Announces Discontinuation of NETest 1.0 in Favor of Advanced NETest 2.0
“Clinicians need a reliable, data-driven tool to make informed treatment decisions,” added Dr. Mark Kidd, Laboratory Director at Wren Laboratories. “With NETest 2.0, we’ve significantly enhanced diagnostic precision, allowing for improved patient surveillance and better treatment outcomes.”
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

A Secure Future for Medical Devices and Patients | By Charles Marrow
While it isn’t possible to completely eliminate the cyber security risk of connected devices, too many of the medical devices we use have security vulnerabilities built-in due to poor design or inherited from 3rd party software.

The Journey to Create a New, Patient-friendly Bladder Control Treatment | By Jill Schiaparelli, Co-Founder and CEO of Avation Medical
Jill realized that there was an opportunity to create an entirely new way to treat OAB and focus on the patient experience – ensuring that the treatment was not only effective but also eliminated the need for drugs and invasive procedures that left scars and required permanent implants.

AI-Powered MSA | By Robert Scott, Chief Innovator — monjur
Robert Scott notes: Crafting an MSA that will address risks and empower growth is a complex process that is constantly evolving. Leveraging AI-powered tools to assist in the process allows businesses to streamline and automate the time-consuming and resource-intensive task. Learn more.
Executives on the Move
Michael Andersen Joins Vektor Medical as Vice President, Quality Assurance and Regulatory Affairs
Michael Andersen brings more than 20 years of commercial leadership experience in electrophysiology and medtech, with a strong track record of market development, sales execution, and strategic growth.
Abbas Kazimi Steps Up as Dynamic CEO of Nimbus Therapeutics
“I am honored to lead Nimbus at this pivotal time in the company’s evolution,” said Abbas Kazimi. “Our robust pipeline, anchored by promising programs like our WRN and SIK inhibitors, and our ongoing collaboration with Lilly, continues to validate our approach and strengthen our position in the industry. I’m particularly excited about what Nimbus will continue to discover and advance, as we leverage our computational drug discovery engine to bring innovative medicines for patients.”
Mirva Ekman Appointed Quality Director & Member of the Management Team at Bioretec
Mirva Ekman over 20 years of experience in quality and regulatory leadership.
FDA Medical Device Updates
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
Hanger Ventures Announces Inaugural Investment
“We are thrilled to make our inaugural investment in Bionic Power,” shared James Campbell, Ph.D., Hanger Ventures President and Hanger, Inc. Chief Clinical Officer. “We look forward to contributing clinical research, along with technical and commercial expertise, to bring innovative solutions to the market that have the potential to meet an unmet clinical need and improve the quality of life for individuals with mobility challenges.”

Novocuff Raises $26 Million in Oversubscribed Series A Funding to Advance Technology Aimed at Reducing Preterm Births
“We are thrilled to have the support of a strong and mission-aligned group of investors as we enter this exciting next phase of clinical development,” said Amelia “Amy” Degenkolb, CEO & Co-founder of Novocuff. “This funding catalyzes our ability to deliver a solution to a healthcare need for women, and their families and healthcare providers.”
Digestiva Closes $18.4 million Series A Financing
Digestiva reports this significant milestone was led by Magdalena, a global leader in sugar cane processing, with participation from UC Investments, and existing investors The March Fund and Astanor. The company’s groundbreaking enzyme technology platform was initially discovered by co-founder Dr. Wilson Mak, in the lab of renowned UC Davis faculty member Dr. Justin Siegel, a global leader in enzyme discovery, development, and design.
Neuspera Medical Raises $23 Million in Series D Financing
Neuspera Medical notes this round will fund the company though expected U.S. Food and Drug Association (FDA) premarket approval (PMA) of the Neuspera System, the discreet, minimally invasive, ultra-miniaturized implant designed to provide patients personal control and relief from urinary urge incontinence (UUI), a symptom of overactive bladder (OAB).
Market Reports
Advancements In Imaging
Perimeter Medical Imaging AI Announces Further Commercial Expansion in North Texas with Follow-On Placement of Perimeter S-Series OCT within National Healthcare Provider System
Adrian Mendes, Perimeter’s Chief Executive Officer, stated, “We believe this additional commercial placement within a major national healthcare system – representing the fifth unit placed with this existing customer network – demonstrates that surgeon end users, as well as other stakeholders within the broader healthcare system, see the value of our technology and are recommending it to their peers. Our aim is to make Perimeter’s innovative technology the standard of care in cancer surgery.”
Vidhance for Remote Assistance Software Suite Transforms Surgeon Training and Expands Global Surgical Access
Vidhance for Remote Assistance not only improves today’s limitations of head-mounted cameras but also contributes to the transformation of how surgical training is conducted.
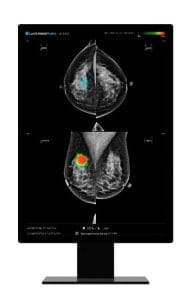
Lunit Advances to Stage 2 of Australia’s National Breast Cancer Screening Project
Last year, Lunit INSIGHT MMG, Lunit’s AI-powered mammography analysis solution, was selected as the preferred AI technology for mammogram screening in the BSNSW State program. The selection of Lunit INSIGHT MMG was a global first, marking the beginning of a new era where national cancer screening organizations assess the operational role of AI solutions in maintaining and improving client outcomes.
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications
Unlocking the Healing Power: The Importance of Nanomedicine
This article covers the place of nanomedicine in modern medicine and how it has altered treatments. Here we also discuss the importance of nanomedicine. Using substances at the nanoscale, like nanoparticles, for medicinal reasons is known as nanomedicine.

Transforming Healthcare with Tailored Digital Solutions
Digital solutions: In the ever-changing landscape of healthcare, custom software solutions have emerged as game-changers, empowering healthcare organizations to achieve unparalleled efficiency, enhance patient experiences, and drive operational excellence. By tailoring digital tools to their unique needs, healthcare providers can streamline workflows, improve collaboration, ensure compliance, and ultimately deliver better outcomes for patients.

5 Ways Healthcare Mobile Apps Are Revolutionizing Patient Care in the Medical Industry
Healthcare mobile apps are revolutionizing patient care in the medical industry.These applications make medical information more accessible, allow for remote consultations and telemedicine, enhance medication administration, aid in the treatment of chronic diseases, and encourage individualized health and well-being.

Telemedicine Business – How to Setup and Stay Afloat
Telemedicine business is not as complicated as many people may think. It is all about taking advantage of the modern technology and incorporating it in the health sector. The most important thing is finding the right telemedicine business software, which you can get through a reliable developer.
Healthcare Careers

8 Benefits Of Considering A Career In Counseling
If you’re considering a career in counseling, you may be wondering what the benefits are. It’s a great question and one that we’re happy to
9 Reasons to Become a Public Health Professional
Public health professionals work to keep the population healthy and elevate the health standards of the communities. All their policies, procedures, and systems are developed

Why Pursuing A Career In Medicine Is A Good Option For Patient People
The field of medicine is one of the most renowned professions in the world. It has a huge advantage and is respected and admired around
Nurses Corner

Reasons Why The Role Of Nurses Is Important In The Healthcare System
Are you thinking of becoming a nurse? Maybe you’re asking yourself why I want to be a nurse. Or are you just curious about the

Easy Stretch by Butter-Soft Scrubs – Comfortable and Flexible Medical Attire
Easy Stretch by Butter-Soft scrubs have revolutionized the medical apparel industry by offering healthcare professionals a comfortable and flexible option for their workwear. These Scrubs
6 Ways Nurses Can Provide Better Patient Care
In healthcare, a nurse can play a crucial role in ensuring that patients receive the highest quality of care. Beyond administering medications and monitoring vital




